Thrombin generation to evaluate the complex hemostatic balance of hemophilia A plasma containing direct oral anticoagulant and supplemented by factor VIII
- PMID: 39498238
- PMCID: PMC11532490
- DOI: 10.1016/j.rpth.2024.102576
Thrombin generation to evaluate the complex hemostatic balance of hemophilia A plasma containing direct oral anticoagulant and supplemented by factor VIII
Abstract
Background: The incidence of cardiovascular diseases is increasing in persons with hemophilia A (HA). Therefore, anticoagulant therapy based on direct oral anticoagulants (DOACs) may be needed, despite the bleeding risk. In case of surgery or bleeding, such patients may be concomitantly treated with emicizumab (routine prophylaxis), factor (F)VIII products, and DOAC. Their concomitant presence constitutes a hemostatic challenge. Recent international guidelines stated that data are scarce on the hemostatic balance of plasma samples from patients with HA receiving emicizumab and DOAC.
Objectives: The aim of this observational study was to assess the coagulation of FVIII-deficient plasma spiked with DOAC and emicizumab and to evaluate the effects of FVIII addition.
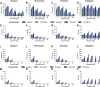
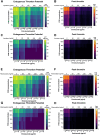
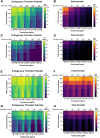
Methods: Prothrombin time, activated partial thromboplastin time, and thrombin generation (TG) using the calibrated automated thrombogram method were evaluated in aliquots of a commercial severe HA plasma supplemented with emicizumab (0, 12.5, 25, 50, and 100 ng/mL), DOAC (0, 50, 100, 200, and 400 ng/mL of apixaban, rivaroxaban, edoxaban, or dabigatran) and FVIII (0%, 5%, 15%, 50%, and 100%).
Results: DOAC rapidly induced a TG decrease. Emicizumab could counter this effect only for the lowest DOAC dose. FVIII addition to the FVIII-deficient plasma containing a DOAC and emicizumab improved TG and countered the anticoagulant effect of DOAC at ≤100 ng/mL.
Conclusion: Our findings indicate that FVIII can be safely used with emicizumab to counter the anticoagulant effect of DOAC at ≤100 ng/mL. The TG assay is an efficient tool to monitor plasma containing anti-FXa DOAC, but not dabigatran (anti-FIIa).
Keywords: direct oral anticoagulant; emicizumab; hemophilia A; monitoring; thrombin.
© 2024 The Authors.
Figures
References
-
- Manco-Johnson M.J., Abshire T.C., Shapiro A.D., Riske B., Hacker M.R., Kilcoyne R., et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–544. - PubMed
-
- Franchini M., Mannucci P.M. The history of hemophilia. Semin Thromb Hemost. 2014;40:571–576. - PubMed
-
- Carcao M.D., Aledort L. Prophylactic factor replacement in hemophilia. Blood Rev. 2004;18:101–113. - PubMed
-
- Schrijvers L.H., Uitslager N., Schuurmans M.J., Fischer K. Barriers and motivators of adherence to prophylactic treatment in haemophilia: a systematic review. Haemophilia. 2013;19:355–361. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

