Synthesis of benzo[ f]quinazoline-1,3(2 H,4 H)-diones
- PMID: 39498449
- PMCID: PMC11533120
- DOI: 10.3762/bjoc.20.228
Synthesis of benzo[ f]quinazoline-1,3(2 H,4 H)-diones
Abstract
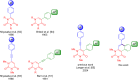
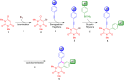
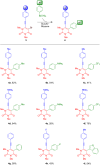
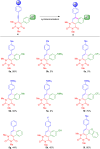
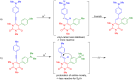
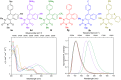
We report the synthesis of polycyclic uracil derivatives. The method is based on palladium-catalysed Sonogashira-Hagihara and Suzuki-Miyaura cross-coupling reactions followed by Brønsted acid-mediated cycloisomerisation. The developed methodology tolerates various functional groups and leads to moderate up to quantitative yields of the final products. The impact of different functional groups on the optical properties was studied by UV-vis and fluorescence spectroscopy.
Keywords: cross-coupling; cyclization; heterocycles; palladium.
Copyright © 2024, de Abreu et al.
Conflict of interest statement
There are no conflicts or financial interests to declare.
Figures

References
-
- Myers R L. The 100 Most Important Chemical Compounds. 1st ed. London, UK: Greenwood Publishing Group; 2007. - DOI
-
- Casy A F, D’Arcy P F, Scott E M, et al. Progress in Drug Research. Vol. 22. Basel, Switzerland: Birkhäuser Verlag; 2013. - DOI
-
- Silverman R B, Holladay M W. The Organic Chemistry of Drug Design and Drug Action. 3rd ed. San Diego, CA, USA: Academic Press; 2015. - DOI
LinkOut - more resources
Full Text Sources
