Phage-Templated Synthesis of Targeted Photoactive 1D-Thiophene Nanoparticles
- PMID: 39498689
- PMCID: PMC11707577
- DOI: 10.1002/smll.202405832
Phage-Templated Synthesis of Targeted Photoactive 1D-Thiophene Nanoparticles
Abstract
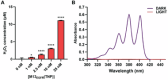
Thiophene-based nanoparticles (TNPs) are promising therapeutic and imaging agents. Here, using an innovative phage-templated synthesis, a strategy able to bypass the current limitations of TNPs in nanomedicine applications is proposed. The phage capsid is decorated with oligothiophene derivatives, transforming the virus in a 1D-thiophene nanoparticle (1D-TNP). A precise control of the shape/size of the nanoparticles is obtained exploiting the well-defined morphology of a refactored filamentous M13 phage, engineered by phage display to selectively recognize the Epidermal Growth Factor Receptor (EGFR). The tropism of the phage is maintained also after the bioconjugation of the thiophene molecules on its capsid. Moreover, the 1D-TNP proved highly fluorescent and photoactive, generating reactive oxygen species through both type I and type II mechanisms. The phototheranostic properties of this platform are investigated on biosystems presenting increasing complexity levels, from in vitro cancer cells in 2D and 3D architectures, to the in vivo tissue-like model organism Hydra vulgaris. The phage-templated 1D-TNP showed photocytotoxicity at picomolar concentrations, and the ability to deeply penetrate 3D spheroids and Hydra tissues. Collectively the results indicate that phage-templated synthesis of organic nanoparticles represents a general strategy, exploitable in many diagnostic and therapeutic fields based on targeted imaging and light mediated cell ablation.
Keywords: M13 phage; photodynamic therapy; phototheranostic platform; thiophene nanoparticles; virus‐templated synthesis.
© 2024 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Perepichka I. F., Perepichka D. F., Handbook of Thiophene‐Based Materials: Applications in Organic Electronics and Photonics, John Wiley and Sons, Hoboken, New Jersey: 2009.
-
- Barbarella G., Zangoli M., Di Maria F., Adv. Heterocycl. Chem. 2017, 123, 105.
-
- Zhang L., Colella N. S., Cherniawski B. P., Mannsfeld S. C. B., Briseno A. L., ACS Appl. Mater. Interfaces 2014, 6, 5327. - PubMed
-
- Fichou D., Handbook of Oligo‐ and Polythiophenes, John Wiley and Sons, Hoboken, New Jersey: 1998.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

