Drying of Soft Colloidal Films
- PMID: 39498779
- PMCID: PMC11653679
- DOI: 10.1002/advs.202406977
Drying of Soft Colloidal Films
Abstract
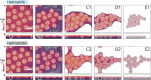
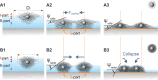
Thin films made of deformable micro- and nano-units, such as biological membranes, polymer interfaces, and particle-laden liquid surfaces, exhibit a complex behavior during drying, with consequences for various applications like wound healing, coating technologies, and additive manufacturing. Studying the drying dynamics and structural changes of soft colloidal films thus holds the potential to yield valuable insights to achieve improvements for applications. In this study, interfacial monolayers of core-shell (CS) microgels with varying degrees of softness are employed as model systems and to investigate their drying behavior on differently modified solid substrates (hydrophobic vs hydrophilic). By leveraging video microscopy, particle tracking, and thin film interference, this study shed light on the interplay between microgel adhesion to solid surfaces and the immersion capillary forces that arise in the thin liquid film. It is discovered that a dried replica of the interfacial microstructure can be more accurately achieved on a hydrophobic substrate relative to a hydrophilic one, particularly when employing softer colloids as opposed to harder counterparts. These observations are qualitatively supported by experiments with a thin film pressure balance which allows mimicking and controlling the drying process and by computer simulations with coarse-grained models.
Keywords: capillary forces; core‐shell microgels; fluid interface‐mediated colloidal assembly; microgel‐to‐substrate adhesion; thin film.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Langevin D., Emulsions, Microemulsions and Foams, Soft and Biological Matter, Springer, Cham; 2020.
-
- Pickering S. U., J. Chem. Soc., Trans. 1907, 91, 2001.
-
- Dekker R. I., Velandia S. F., Kibbelaar H. V. M., Morcy A., Sadtler V., Roques‐Carmes T., Groenewold J., Kegel W. K., Velikov K. P., Bonn D., Soft Matter 2023, 19, 1941. - PubMed
-
- Binks B. P., Curr. Opin. Colloid Interface Sci. 2002, 7, 21.
-
- Zeng C., Faaborg M. W., Sherif A., Falk M. J., Hajian R., Xiao M., Hartig K., Bar‐Sinai Y., Brenner M. P., Manoharan V. N., Nature 2022, 611, 68. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
