Photooxidation of Nonanoic Acid by Molecular and Complex Environmental Photosensitizers
- PMID: 39498797
- PMCID: PMC11571206
- DOI: 10.1021/acs.jpca.4c05608
Photooxidation of Nonanoic Acid by Molecular and Complex Environmental Photosensitizers
Abstract
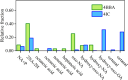
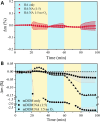
Photochemical aging and photooxidation of atmospheric particles play a crucial role in both the chemical and physical processes occurring in the troposphere. In particular, the presence of organic chromophores within atmospheric aerosols can trigger photosensitized oxidation that drives the atmospheric processes in these interfaces. However, the light-induced oxidation of the surface of atmospheric aerosols, especially those enriched with organic components, remains poorly understood. Herein, we present a gravimetric and vibrational spectroscopy study aimed to investigate the photosensitized oxidation of nonanoic acid (NA), a model system of fatty acids within organic aerosols, in the presence of complex organic photosensitizers and molecular proxies. Specifically, this study shows a comparative analysis of the photosensitized reactions of thin films containing nonanoic acid and four different organic photosensitizers, namely marine dissolved organic matter (m-DOM) and humic acids (HA) as environmental photosensitizers, and 4-imidazolecarboxaldehyde (4IC) and 4-benzoylbenzoic acid (4BBA) as molecular proxies. All reactions show predominant photooxidation of nonanoic acid, with important differences in the rate and yield of product formation depending on the photosensitizer. Limited changes were observed in the organic photosensitizer itself. Results show that, among the photosensitizers examined, 4BBA is the most effective in photooxidizing nonanoic acid. Overall, this work underscores the role of chromophores in the photooxidation of organic thin films and the relevance of such reactions on the surface of aerosols in the marine environment.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Trueblood J. V.; Wang X.; Or V. W.; Alves M. R.; Santander M. V.; Prather K. A.; Grassian V. H. The Old and the New: Aging of Sea Spray Aerosol and Formation of Secondary Marine Aerosol through OH Oxidation Reactions. ACS Earth Space Chem. 2019, 3, 2307–2314. 10.1021/acsearthspacechem.9b00087. - DOI
-
- Navea J. G.; Grassian V. H.. Photochemistry of Atmospheric Particles. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt K.; Kolasinski K., Eds.; Elsevier Publishing: Amsterdam, 2018; pp 553–562.
-
- Leonardi A.; Ricker H. M.; Gale A. G.; Ball B. T.; Odbadrakh T. T.; Shields G. C.; Navea J. G. Particle Formation and Surface Processes on Atmospheric Aerosols: A Review of Applied Quantum Chemical Calculations. Int. J. Quantum Chem. 2020, 120, e26350 10.1002/qua.26350. - DOI
LinkOut - more resources
Full Text Sources

