Studying bats using a One Health lens: bridging the gap between bat virology and disease ecology
- PMID: 39499009
- PMCID: PMC11650978
- DOI: 10.1128/jvi.01453-24
Studying bats using a One Health lens: bridging the gap between bat virology and disease ecology
Abstract
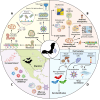
Accumulating data suggest that some bat species host emerging viruses that are highly pathogenic in humans and agricultural animals. Laboratory-based studies have highlighted important adaptations in bat immune systems that allow them to better tolerate viral infections compared to humans. Simultaneously, ecological studies have discovered critical extrinsic factors, such as nutritional stress, that correlate with virus shedding in wild-caught bats. Despite some progress in independently understanding the role of bats as reservoirs of emerging viruses, there remains a significant gap in the molecular understanding of factors that drive virus spillover from bats. Driven by a collective goal of bridging the gap between the fields of bat virology, immunology, and disease ecology, we hosted a satellite symposium at the 2024 American Society for Virology meeting. Bringing together virologists, immunologists, and disease ecologists, we discussed the intrinsic and extrinsic factors such as virus receptor engagement, adaptive immunity, and virus ecology that influence spillover from bat hosts. This article summarizes the topics discussed during the symposium and emphasizes the need for interdisciplinary collaborations and resource sharing.
Keywords: ASV 2024; bats; disease ecology; immunity; satellite symposium; virology.
Conflict of interest statement
A.B. is a co-inventor of the Efk3B cell line that is sold through Kerafast, USA. All other authors declare no conflicts of interest.
Figures
References
-
- Burgin CJ, Colella JP, Kahn PL, Upham NS. 2018. How many species of mammals are there? J Mammal 99:1–14. doi:10.1093/jmammal/gyx147 - DOI
-
- Schuh AJ, Amman BR, Jones MEB, Sealy TK, Uebelhoer LS, Spengler JR, Martin BE, Coleman-McCray JAD, Nichol ST, Towner JS. 2017. Modelling filovirus maintenance in nature by experimental transmission of Marburg virus between Egyptian rousette bats. Nat Commun 8:14446. doi:10.1038/ncomms14446 - DOI - PMC - PubMed
-
- Halpin K, Hyatt AD, Fogarty R, Middleton D, Bingham J, Epstein JH, Rahman SA, Hughes T, Smith C, Field HE, Daszak P, Henipavirus Ecology Research Group . 2011. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: a comprehensive experimental study of virus transmission. Am J Trop Med Hyg 85:946–951. doi:10.4269/ajtmh.2011.10-0567 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

