Extracellular vesicles from human-induced pluripotent stem cell-derived neural stem cells alleviate proinflammatory cascades within disease-associated microglia in Alzheimer's disease
- PMID: 39499013
- PMCID: PMC11536387
- DOI: 10.1002/jev2.12519
Extracellular vesicles from human-induced pluripotent stem cell-derived neural stem cells alleviate proinflammatory cascades within disease-associated microglia in Alzheimer's disease
Abstract
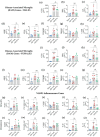
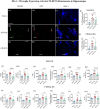
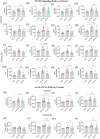
As current treatments for Alzheimer's disease (AD) lack disease-modifying interventions, novel therapies capable of restraining AD progression and maintaining better brain function have great significance. Anti-inflammatory extracellular vesicles (EVs) derived from human induced pluripotent stem cell (hiPSC)-derived neural stem cells (NSCs) hold promise as a disease-modifying biologic for AD. This study directly addressed this issue by examining the effects of intranasal (IN) administrations of hiPSC-NSC-EVs in 3-month-old 5xFAD mice. IN administered hiPSC-NSC-EVs incorporated into microglia, including plaque-associated microglia, and encountered astrocyte soma and processes in the brain. Single-cell RNA sequencing revealed transcriptomic changes indicative of diminished activation of microglia and astrocytes. Multiple genes linked to disease-associated microglia, NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3)-inflammasome and interferon-1 (IFN-1) signalling displayed reduced expression in microglia. Adding hiPSC-NSC-EVs to cultured human microglia challenged with amyloid-beta oligomers resulted in similar effects. Astrocytes also displayed reduced expression of genes linked to IFN-1 and interleukin-6 signalling. Furthermore, the modulatory effects of hiPSC-NSC-EVs on microglia in the hippocampus persisted 2 months post-EV treatment without impacting their phagocytosis function. Such effects were evidenced by reductions in microglial clusters and inflammasome complexes, concentrations of mediators, and end products of NLRP3 inflammasome activation, the expression of genes and/or proteins involved in the activation of p38/mitogen-activated protein kinase and IFN-1 signalling, and unaltered phagocytosis function. The extent of astrocyte hypertrophy, amyloid-beta plaques, and p-tau were also reduced in the hippocampus. Such modulatory effects of hiPSC-NSC-EVs also led to better cognitive and mood function. Thus, early hiPSC-NSC-EV intervention in AD can maintain better brain function by reducing adverse neuroinflammatory signalling cascades, amyloid-beta plaque load, and p-tau. These results reflect the first demonstration of the efficacy of hiPSC-NSC-EVs to restrain neuroinflammatory signalling cascades in an AD model by inducing transcriptomic changes in activated microglia and reactive astrocytes.
Keywords: Anti‐inflammatory effects; disease‐associated microglia; extracellular vesicles; human induced pluripotent stem cell‐derived neural stem cells; inflammasomes; interferon 1 signalling; mitogen‐activated protein kinase signalling.
© 2024 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


Update of
-
Intranasally Administered EVs from hiPSC-derived NSCs Alter the Transcriptomic Profile of Activated Microglia and Conserve Brain Function in an Alzheimer's Model.bioRxiv [Preprint]. 2024 Jan 19:2024.01.18.576313. doi: 10.1101/2024.01.18.576313. bioRxiv. 2024. Update in: J Extracell Vesicles. 2024 Nov;13(11):e12519. doi: 10.1002/jev2.12519. PMID: 38293018 Free PMC article. Updated. Preprint.
References
-
- Akama, K. T. , & Van Eldik, L. J. (2000). Beta‐amyloid stimulation of inducible nitric‐oxide synthase in astrocytes is interleukin‐1beta‐ and tumor necrosis factor‐alpha (TNFalpha)‐dependent, and involves a TNFalpha receptor‐associated factor‐ and NFkappaB‐inducing kinase‐dependent signalling mechanism. Journal of Biological Chemistry, 275, 7918–7924. 10.1074/jbc.275.11.7918 - DOI - PubMed
-
- Apodaca, L. A. , Baddour, A. A. D. , Garcia, C. , Jr., Alikhani, L. , Giedzinski, E. , Ru, N. , Agrawal, A. , Acharya, M. M. , & Baulch, J. E. (2021). Human neural stem cell‐derived extracellular vesicles mitigate hallmarks of Alzheimer's disease. Alzheimers Research & Therapy, 13, 57. 10.1186/s13195-021-00791-x - DOI - PMC - PubMed
-
- Attaluri, S. , Jaimes Gonzalez, J. , Kirmani, M. , Vogel, A. D. , Upadhya, R. , Kodali, M. , Madhu, L. N. , Rao, S. , Shuai, B. , Babu, R. S. , Huard, C. , & Shetty, A. K. (2023). Intranasally administered extracellular vesicles from human induced pluripotent stem cell‐derived neural stem cells quickly incorporate into neurons and microglia in 5xFAD mice. Frontiers in Aging Neuroscience, 15, 1200445. 10.3389/fnagi.2023.1200445 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

