Disease Characteristics and Outcomes of 493 Young Myeloma Patients Treated With Modern Therapies: A Canadian Myeloma Research Group Database Study
- PMID: 39499045
- PMCID: PMC11536480
- DOI: 10.1002/cam4.70332
Disease Characteristics and Outcomes of 493 Young Myeloma Patients Treated With Modern Therapies: A Canadian Myeloma Research Group Database Study
Abstract
Background: Young patients ≤ 50 years old with multiple myeloma (MM) account for about 10% of cases and are underrepresented in the literature.
Methods: We explored disease characteristics, treatments, and outcomes following modern therapies of young MM patients using the Canadian Myeloma Research Group (CMRG) database. We included 493 patients ≤ 50 years old diagnosed with MM or plasma cell leukemia without concurrent amyloidosis or POEMS syndrome from January 1, 2010, to July 1, 2022.
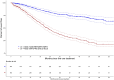
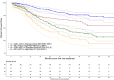
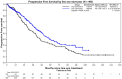
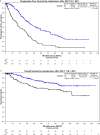
Results: The median age was 46 years old (range: 25.6-50). Most patients fell into the R-ISS II category (72.7%), and 24.1% had high-risk cytogenetics. The majority of patients (89.9%) received a proteasome inhibitor-based first-line treatment, 92.1% received a stem cell transplant, and 65.6% had maintenance therapy post-autologous stem cell transplant (ASCT). Median follow-up from initial treatment to patients' last follow-up was 48.5 (range: 0-155) months. Median progression-free survival (PFS) was 45.0 months (95% CI: 40.2-50.0). Maintenance therapy post-ASCT improved median PFS to 52.3 months (95% CI: 43.1-68.2), compared to 23.6 months (95% CI: 20.0-34.8) without maintenance [p < 0.001].
Conclusion: Although the overall survival has not yet been reached in this young population, our reported median PFS of only 45 months highlights the urgent need to develop innovative treatments to induce more profound and durable responses.
Keywords: chemotherapy; clinical observations; epidemiology; multiple myeloma; registries; survival.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
M.T. declares no competing interests. J.R. has received honoraria from Janssen and was part of advisory committees for Sanofi, Amgen, and Janssen. He received grant support and had royalties from ExCellThera. D.R. has received honoraria from BMS, Janssen, Takeda, Sanofi, Pfizer, and GSK. She served as a consultant for Janssen, Amgen, Takeda, and BMS. She received research funding from Janssen, Takeda, BMS, and Millennium. C.P.V. has received honoraria from Janssen, BMS, Pfizer, Abbvie, Sanofi, Forus, and GSK. D.W. has received honoraria from Amgen, Antengene, BMS, Forus, GSK, Janssen, Karyopharm, Pfizer, Sanofi, and Takeda. M.P.C. has received honoraria from AstraZeneca, BMS/Celgene, Gilead, Janssen, AbbVie, Pfizer, Sanofi, and Amgen. He received research funding from BMS/Celgene and Miltenyi. V.H.J‐Z. has received honoraria from Celgene, Janssen, Takeda, Merck, and BMS. K.S. has received honoraria from BMS, Janssen, Amgen, and Sanofi. A.M. has received honoraria from Celgene, Janssen, Amgen, Takeda, Sanofi, and GSK. H.M. has received honoraria from Celgene, Janssen, Amgen, Takeda, and Sanofi. She received the GSK Awards: HHS Research Early Career Award from the Hamilton Health Sciences Foundation. M.S. had membership on an entity's Board of Directors or advisory committees for Janssen, Amgen, Takeda, and Celgene. D.B. has received honoraria from Janssen and BMS. She received research funding from BMS. J.S. has received honoraria from Janssen, FORUS Therapeutics, BMS, Pfizer, Sanofi, and Amgen. A.R. served as a consultant and received honoraria and research funding from Janssen, Sanofi, BMS, Takeda, Pfizer, Regeneron, and AstraZeneca. R. Ko. has received honoraria from Akcea, Amgen, BMS, Janssen, Merck, Sanofi, Celgene, Pfizer, and Takeda. He has received research funding from Merck and Sanofi and holds equity in the private company Karyopharm. M.A. has received honoraria from AbbVie, Gilead, Janssen, and Celgene. R.Ka. has received honoraria from Janssen, BMS, FORUS, Sanofi, and Pfizer. M.L. has received honoraria from Janssen, Celgene, Amgen, and Pfizer. R.L. received research funding from Amgen and Sanofi, served as a consultant for BMS, Janssen, Amgen, Sanofi, and FORUS Therapeutics, and received honoraria from Pfizer and Janssen.
Figures
References
-
- National Cancer Institute , “SEER Cancer Statistics Factsheets: Multiple Myeloma,” https://seer.cancer.gov/statfacts/html/mulmy.html.
-
- Ludwig H., Bolejack V., Crowley J., et al., “Survival and Years of Life Lost in Different Age Cohorts of Patients With Multiple Myeloma,” Journal of Clinical Oncology 28, no. 9 (2010): 1599–1605. - PubMed
-
- van der Poel M. W. M., Oerlemans S., Schouten H. C., and van de Poll‐Franse L. V., “Elderly Multiple Myeloma Patients Experience Less Deterioration in Health‐Related Quality of Life Than Younger Patients Compared to a Normative Population: A Study From the Population‐Based PROFILES Registry,” Annals of Hematology 94, no. 4 (2015): 651–661. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

