SUMO Regulation of Ion Channels in Health and Disease
- PMID: 39499247
- PMCID: PMC12183622
- DOI: 10.1152/physiol.00034.2024
SUMO Regulation of Ion Channels in Health and Disease
Abstract
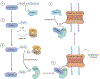
The small ubiquitin-like modifier (SUMO) protein pathway governs a panoply of vital biological processes including cell death, proliferation, differentiation, metabolism, and signal transduction by diversifying the functions, half-lives, and partnerships of target proteins in situ. More recently, SUMOylation has emerged as a key regulator of ion homeostasis and excitability across multiple tissues due to the regulation of a plethora of ion channels expressed in a range of tissue subtypes. Altogether, the balance of SUMOylation states among relevant ion channels can result in graded biophysical effects that tune excitability and contribute to a range of disease states including cardiac arrhythmia, epilepsy, pain transmission, and inflammation. Here, we consolidate these concepts by focusing on the role of ion channel SUMOylation in the central nervous system, peripheral nervous system, and cardiovascular system. In addition, we review what is known about the enigmatic factors that regulate the SUMO pathway and consider the emerging role of small molecule SUMO modulators as potential therapeutics in a range of diseases.
Keywords: SUMOylation; UbL; Ubc9; ion channels; posttranslational modification.
Conflict of interest statement
Disclosures
The authors declare that they have no conflicts of interest with the contents of this article.
Figures
References
-
- Hershko A, and Ciechanover A. The ubiquitin system. Annu Rev Biochem 67: 425–479, 1998. - PubMed
-
- van der Veen AG, and Ploegh HL. Ubiquitin-like proteins. Annu Rev Biochem 81: 323–357, 2012. - PubMed
-
- Delley CL, Muller AU, Ziemski M, and Weber-Ban E. Prokaryotic Ubiquitin-Like Protein and Its Ligase/Deligase Enyzmes. J Mol Biol 429: 3486–3499, 2017. - PubMed
-
- Mahajan R, Delphin C, Guan T, Gerace L, and Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88: 97–107, 1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

