Ocular biometric responses to simulated polychromatic defocus
- PMID: 39499528
- PMCID: PMC11540029
- DOI: 10.1167/jov.24.12.3
Ocular biometric responses to simulated polychromatic defocus
Abstract
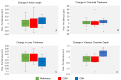
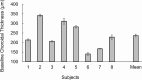
Evidence from human studies of ocular accommodation and studies of animals reared in monochromatic conditions suggest that chromatic signals can guide ocular growth. We hypothesized that ocular biometric response in humans can be manipulated by simulating the chromatic contrast differences associated with imposition of optical defocus. The red, green, and blue (RGB) channels of an RGB movie of the natural world were individually incorporated with computational defocus to create two different movie stimuli. The magnitude of defocus incorporated in the red and blue layers was chosen such that, in one case, it simulated +3 D defocus, referred to as color-signed myopic (CSM) defocus, and in another case it simulated -3 D defocus, referred to as color-signed hyperopic (CSH) defocus. Seventeen subjects viewed the reference stimulus (unaltered movie) and at least one of the two color-signed defocus stimuli for ∼1 hour. Axial length (AL) and choroidal thickness (ChT) were measured immediately before and after each session. AL and subfoveal ChT showed no significant change under any of the three conditions. A significant increase in vitreous chamber depth (VCD) was observed following viewing of the CSH stimulus compared with the reference stimulus (0.034 ± 0.03 mm and 0 ± 0.02 mm, respectively; p = 0.018). A significant thinning of the crystalline lens was observed following viewing of the CSH stimulus relative to the CSM stimulus (-0.033 ± 0.03 mm and 0.001 ± 0.03 mm, respectively; p = 0.015). Differences in the effects of CSM and CSH conditions on VCD and lens thickness suggest a directional, modulatory influence of chromatic defocus. On the other hand, ChT responses showed large variability, rendering it an unreliable biomarker for chromatic defocus-driven responses, at least for the conditions of this study.
Figures





References
-
- Aldakhil, S. (2021). The effect of optical defocus on the choroidal thickness: A review. The Open Ophthalmology Journal, 15(1), 283–287, 10.2174/1874364102115010283. - DOI
-
- Arumugam, B., Hung, L. F., To, C. H., Sankaridurg, P., & Smith, E. L. (2016). The effects of the relative strength of simultaneous competing defocus signals on emmetropization in infant rhesus monkeys. Investigative Ophthalmology and Visual Science, 57(10), 3949–3960, 10.1167/iovs.16-19704. - DOI - PMC - PubMed

