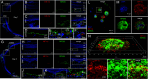
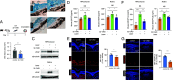
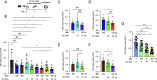
VEGF inhibition increases expression of HIF-regulated angiogenic genes by the RPE limiting the response of wet AMD eyes to aflibercept
- PMID: 39499641
- PMCID: PMC11573522
- DOI: 10.1073/pnas.2322759121
VEGF inhibition increases expression of HIF-regulated angiogenic genes by the RPE limiting the response of wet AMD eyes to aflibercept
Abstract
Neovascular age-related macular degeneration (nvAMD) is the leading cause of severe vision loss in the elderly in the developed world. While the introduction of therapies targeting vascular endothelial growth factor (VEGF) has provided the first opportunity to significantly improve vision in patients with nvAMD, many patients respond inadequately to current anti-VEGF therapies. It was recently demonstrated that expression of a second angiogenic mediator, angiopoietin-like 4 (ANGPTL4), synergizes with VEGF to promote choroidal neovascularization (CNV) in mice and correlates with reduced response to anti-VEGF therapy in patients with nvAMD. Here, we report that expression of ANGPTL4 in patients with nvAMD increases following treatment with anti-VEGF therapy and that this increase is dependent on accumulation of hypoxia-inducible factor (HIF)-1α in response to inhibition of VEGF/KDR signaling in the retinal pigment epithelium (RPE). We therefore explored HIF-1 inhibition with 32-134D, a recently developed pharmacologic HIF-inhibitor, for the treatment of nvAMD. 32-134D prevented the expression of both VEGF and ANGPTL4 and was at least as effective as aflibercept in treating CNV in mice. Moreover, by preventing the increase in HIF-1α accumulation in the RPE in response to anti-VEGF therapy, combining 32-134D with aflibercept was more effective than either drug alone for the treatment of CNV. Collectively, these results help explain why many patients with nvAMD respond inadequately to anti-VEGF therapy and suggest that the HIF inhibitor 32-134D will be an effective drug-alone or in combination with current anti-VEGF therapies-for the treatment of patients with this blinding disease.
Keywords: age-related macular degeneration; angiopoietin-like 4; choroidal neovascularization; hypoxia inducible factor; vascular endothelial growth factor.
Conflict of interest statement
Competing interests statement:G.L.S. and A.S. are co-founders of and hold equity in HIF Therapeutics, Inc. S.S., Y.H., G.L.S., and A.S. are inventors on provisional patent application PCT/US2022/039883. This arrangement has been reviewed and approved by the Johns Hopkins University in accordancewith its conflict of interest policies.
Figures