Hyperglycemia-Enhanced Neutrophil Extracellular Traps Drive Mucosal Immunopathology at the Oral Barrier
- PMID: 39499780
- PMCID: PMC11653653
- DOI: 10.1002/advs.202407346
Hyperglycemia-Enhanced Neutrophil Extracellular Traps Drive Mucosal Immunopathology at the Oral Barrier
Abstract
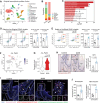
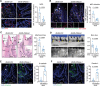
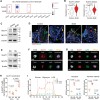
Type 2 diabetes (T2D) is a risk factor for mucosal homeostasis and enhances the susceptibility to inflammation, in which neutrophils have been increasingly appreciated for their role. Here, barrier disruption and inflammation are observed at oral mucosa (gingiva) of T2D patients and mice. It is demonstrated that neutrophils infiltrate the gingival mucosa of T2D mice and expel obvious neutrophil extracellular traps (NETs), while removal of NETs alleviates the disruption of mucosal barrier. Mechanistically, gingival neutrophils released NETs are dependent of their metabolic reprogramming. Under hyperglycemic condition, neutrophils elevate both glucose incorporation and glycolysis via increased expression of GLUT1. Moreover, significantly increased levels of NETs are observed in local gingival lesions of patients, which are associated with clinical disease severity. This work elucidates a causative link between hyperglycemia and oral mucosal immunopathology, mediated by the altered immuno-metabolic axis in neutrophil, thereby suggesting a potential therapeutic strategy.
Keywords: glucose transporter 1; glycolysis; hyperglycemia; neutrophils; oral mucosal immunity; type 2 diabetes.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Baicalin mitigates hyperglycemia-linked intestinal epithelial barrier impairment in part by inhibiting the formation of neutrophil extracellular traps.Front Immunol. 2025 Mar 3;16:1551256. doi: 10.3389/fimmu.2025.1551256. eCollection 2025. Front Immunol. 2025. PMID: 40098957 Free PMC article.
-
Single-cell atlas of human gingiva unveils a NETs-related neutrophil subpopulation regulating periodontal immunity.J Adv Res. 2025 Jun;72:287-301. doi: 10.1016/j.jare.2024.07.028. Epub 2024 Jul 30. J Adv Res. 2025. PMID: 39084404 Free PMC article.
-
Glucose induces metabolic reprogramming in neutrophils during type 2 diabetes to form constitutive extracellular traps and decreased responsiveness to lipopolysaccharides.Biochim Biophys Acta Mol Basis Dis. 2020 Dec 1;1866(12):165940. doi: 10.1016/j.bbadis.2020.165940. Epub 2020 Aug 20. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32827651
-
Neutrophils Orchestrate the Periodontal Pocket.Front Immunol. 2021 Nov 24;12:788766. doi: 10.3389/fimmu.2021.788766. eCollection 2021. Front Immunol. 2021. PMID: 34899756 Free PMC article. Review.
-
Contemporary Lifestyle and Neutrophil Extracellular Traps: An Emerging Link in Atherosclerosis Disease.Cells. 2021 Aug 4;10(8):1985. doi: 10.3390/cells10081985. Cells. 2021. PMID: 34440753 Free PMC article. Review.
Cited by
-
Glycemic control and vaccine response: the role of mucosal immunity after vaccination in diabetic patients.Front Immunol. 2025 May 8;16:1577523. doi: 10.3389/fimmu.2025.1577523. eCollection 2025. Front Immunol. 2025. PMID: 40406123 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
