Comparative Proteomics Highlights that GenX Exposure Leads to Metabolic Defects and Inflammation in Astrocytes
- PMID: 39499804
- PMCID: PMC11580177
- DOI: 10.1021/acs.est.4c05472
Comparative Proteomics Highlights that GenX Exposure Leads to Metabolic Defects and Inflammation in Astrocytes
Abstract
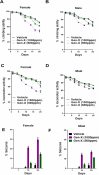
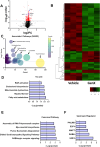
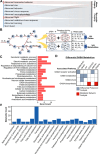
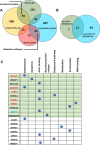
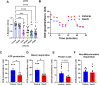
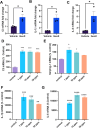
Exposure to PFAS such as GenX (HFPO dimer acid) has become increasingly common due to the replacement of older generation PFAS in manufacturing processes. While neurodegenerative and developmental effects of legacy PFAS exposure have been studied in depth, there is a limited understanding specific to the effects of GenX exposure. To investigate the effects of GenX exposure, we exposed Drosophila melanogaster to GenX and assessed the motor behavior and performed quantitative proteomics of fly brains to identify molecular changes in the brain. Additionally, metabolic network-based analysis using the iDrosophila1 model unveiled a potential link between GenX exposure and neurodegeneration. Since legacy PFAS exposure has been linked to Parkinson's disease (PD), we compared the proteome data sets between GenX-exposed flies and a fly model of PD expressing human α-synuclein. Considering the proteomic data- and network-based analyses that revealed GenX may be regulating GABA-associated pathways and the immune system, we next explored the effects of GenX on astrocytes, as astrocytes in the brain can regulate GABA. An array of assays demonstrated GenX exposure may lead to mitochondrial dysfunction and neuroinflammatory response in astrocytes, possibly linking non-cell autonomous neurodegeneration to the motor deficits associated with GenX exposure.
Keywords: Drosophila; HFPO-DA; astrocytes; behavior; nontargeted proteomics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Foguth R. M.; Hoskins T. D.; Clark G. C.; Nelson M.; Flynn R. W.; de Perre C.; Hoverman J. T.; Lee L. S.; Sepulveda M. S.; Cannon J. R. Single and mixture per- and polyfluoroalkyl substances accumulate in developing Northern leopard frog brains and produce complex neurotransmission alterations. Neurotoxicol. Teratol. 2020, 81, 106907. 10.1016/j.ntt.2020.106907. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

