GnRH-Gonadotropes Interactions Revealed by Pituitary Single-cell Transcriptomics in Zebrafish
- PMID: 39499852
- PMCID: PMC11565244
- DOI: 10.1210/endocr/bqae151
GnRH-Gonadotropes Interactions Revealed by Pituitary Single-cell Transcriptomics in Zebrafish
Erratum in
-
Correction to: "GnRH-Gonadotropes Interactions Revealed by Pituitary Single-cell Transcriptomics in Zebrafish".Endocrinology. 2025 May 19;166(7):bqaf098. doi: 10.1210/endocr/bqaf098. Endocrinology. 2025. PMID: 40457745 Free PMC article. No abstract available.
Abstract
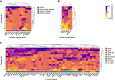
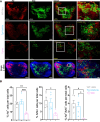
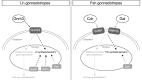
GnRH governs reproduction by regulating pituitary gonadotropins. Unlike most vertebrates, gnrh-/- zebrafish are fertile. To elucidate the role of the hypophysiotropic-Gnrh3 and other mechanisms regulating pituitary gonadotropes, we profiled the gene expression of all individual pituitary cells of wild-type and gnrh3-/- adult female zebrafish. The single-cell RNA sequencing showed that LH and FSH gonadotropes express the 2 gonadotropin beta subunits with a ratio of 140:1 (lhb:fshb) and 4:1 (fshb:lhb), respectively. Lh gonadotropes predominantly express genes encoding receptors for GnRH (gnrhr2), thyroid hormone, estrogen, and steroidogenic factor 1. No GnRH receptor transcript was enriched in FSH gonadotropes. Instead, cholecystokinin receptor-b and galanin receptor-1b transcripts were enriched in these cells. The loss of the Gnrh3 gene in gnrh3-/- zebrafish resulted in downregulation of fshb in LH gonadotropes and upregulation of pituitary hormones like TSH, GH, prolactin, and proopiomelanocortin-a. Likewise, targeted chemogenetic ablation of Gnrh3 neurons led to a decrease in the number of fshb+, lhb + and fshb+/lhb + cells. Our studies suggest that Gnrh3 directly acts on LH gonadotropes through Gnrhr2, but the outcome of this interaction is still unknown. Gnrh3 also regulates fshb expression in both gonadotropes, most likely via a non-GnRH receptor route. Altogether, while LH secretion and synthesis are likely regulated in a GnRH-independent manner, Gnrh3 seems to play a role in the cellular organization of the pituitary. Moreover, the coexpression of lhb and fshb in both gonadotropes provides a possible explanation as to why gnrh3-/- zebrafish are fertile.
Keywords: GnRH; LH; pituitary; reproduction; teleost; transcriptomics.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
-
- Levavi-Sivan B, Bogerd J, Mañanós EL, Gómez A, Lareyre JJ. Perspectives on fish gonadotropins and their receptors. Gen Comp Endocrinol. 2010;165(3):412‐437. - PubMed
-
- Kaprara T, Huhtaniemi IT. The hypothalamus-pituitary-gonad axis: tales of mice and men. Metabolism. 2018;86:3‐17. - PubMed
-
- Colledge WH. The neuroendocrine regulation of the mammalianreproductive axis. Exp Physiol. 2013;98(11):1519‐1521. - PubMed
-
- Bouligand J, Ghervan C, Guiochon-Mantel A, Young J. Hypogonadotropic hypogonadism and GNRH1 mutations in mice and humans. Front Horm Res. 2010;39:111‐120. - PubMed
-
- Bouligand JCG, Trabado S, Brailly-Tabard S, Guiochon-Mantel A, Young J. Genetics defects in GNRH1: a paradigm of hypothalamic congenital gonadotropin deficiency. Brain Res. 2010;1364:3‐9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

