Molecular Tumor Board of the University Medical Center Groningen (UMCG-MTB): outcome of patients with rare or complex mutational profiles receiving MTB-advised targeted therapy
- PMID: 39500140
- PMCID: PMC11570463
- DOI: 10.1016/j.esmoop.2024.103966
Molecular Tumor Board of the University Medical Center Groningen (UMCG-MTB): outcome of patients with rare or complex mutational profiles receiving MTB-advised targeted therapy
Abstract
Purpose: Molecular tumor boards (MTBs) are considered beneficial for treatment decision making for patients with cancer with uncommon, rare, or complex mutational profiles. The lack of international MTB guidelines results in significant variation in practices and recommendations. Therefore, periodic follow-up is necessary to assess and govern MTB functioning. The objective of this study was to determine the effectiveness of MTB treatment recommendations for patients with rare and complex mutational profiles as implemented in the MTB of the University Medical Center Groningen (UMCG-MTB) in 2019-2020.
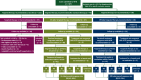
Patients and methods: A retrospective follow-up study was carried out to determine the clinical outcome of patients with uncommon or rare (combinations of) molecular aberrations for whom targeted therapy was recommended as the next line of treatment by the UMCG-MTB in 2019 and 2020.
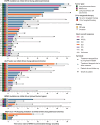
Results: The UMCG-MTB recommended targeted therapy as the next line of treatment in 132 of 327 patients: 37 in clinical trials, 67 in the on-label setting, and 28 in the off-label setting. For on- and off-label treatment recommendations, congruence of recommended and received treatment was 85% in patients with available follow-up (67/79). Treatment with on-label therapy resulted in a response rate of 50% (21/42), a median progression-free survival (PFS) of 6.3 months [interquartile range (IQR) 2.9-14.9 months], and median overall survival (OS) of 15.8 months (IQR 6.4-34.2 months). Treatment with off-label therapy resulted in a response rate of 53% (8/15), a median PFS of 5.1 months (IQR 1.9-7.3 months), and a median OS of 17.7 months (IQR 5.1-23.7 months).
Conclusion: Treatment with MTB-recommended next-line targeted therapy for patients with often heavily pretreated cancer with rare and complex mutational profiles resulted in positive overall responses in over half of patients. Off-label use of targeted therapies, for which there is sufficient rationale as determined by an MTB, is an effective treatment strategy. This study underlines the relevance of discussing patients with rare and complex mutational profiles in an MTB.
Keywords: clinical decision making; molecular pathology; molecular tumor board; precision oncology; real-world data; targeted therapy.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- van der Velden D.L., van Herpen C.M.L., van Laarhoven H.W.M., et al. Molecular tumor boards: current practice and future needs. Ann Oncol. 2017;28(12):3070–3075. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

