Cancer-associated fibroblasts shape early myeloid cell response to chemotherapy-induced immunogenic signals in next generation tumor organoid cultures
- PMID: 39500527
- PMCID: PMC11535717
- DOI: 10.1136/jitc-2024-009494
Cancer-associated fibroblasts shape early myeloid cell response to chemotherapy-induced immunogenic signals in next generation tumor organoid cultures
Abstract
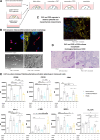
Background: Patient-derived colorectal cancer (CRC) organoids (PDOs) solely consisting of malignant cells led to major advances in the understanding of cancer treatments. Yet, a major limitation is the absence of cells from the tumor microenvironment, thereby prohibiting potential investigation of treatment responses on immune and structural cells. Currently there are sparse reports describing the interaction of PDOs, cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs) in complex primary co-culture assay systems.
Methods: Primary PDOs and patient matched CAF cultures were generated from surgical resections. Co-culture systems of PDOs, CAFs and monocytic myeloid cells were set up to recapitulate features seen in patient tumors. Single-cell transcriptomics and flow cytometry was used to show effects of culture systems on TAM populations in the co-culture assays under chemotherapeutic and oncolytic viral treatment.
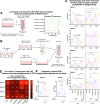
Results: In contrast to co-cultures of tumor cells and monocytes, CAF/monocyte co-cultures and CAF/monocyte/tumor cell triple cultures resulted in a partial differentiation into macrophages and a phenotypic switch, characterized by the expression of major immunosuppressive markers comparable to TAMs in CRC. Oxaliplatin and 5-fluorouracil, the standard-of-care chemotherapy for CRC, induced polarization of macrophages to a pro-inflammatory phenotype comparable to the immunogenic effects of treatment with an oncolytic virus. Monitoring phagocytosis as a functional proxy to macrophage activation and subsequent onset of an immune response, revealed that chemotherapy-induced cell death, but not virus-mediated cell death, is necessary to induce phagocytosis of CRC cells. Moreover, CAFs enhanced the phagocytic activity in chemotherapy treated CRC triple cultures.
Conclusions: Primary CAF-containing triple cultures successfully model TAM-like phenotypes ex vivo and allow the assessment of their functional and phenotypic changes in response to treatments following a precision medicine approach.
Keywords: Cancer associated fibroblasts; Chemotherapy; Colorectal Cancer; Organoids; Tumor Associated Macrophages.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
