Intratumoral and peritumoral radiomics of MRIs predicts pathologic complete response to neoadjuvant chemoimmunotherapy in patients with head and neck squamous cell carcinoma
- PMID: 39500529
- PMCID: PMC11552555
- DOI: 10.1136/jitc-2024-009616
Intratumoral and peritumoral radiomics of MRIs predicts pathologic complete response to neoadjuvant chemoimmunotherapy in patients with head and neck squamous cell carcinoma
Abstract
Background: For patients with locally advanced head and neck squamous cell carcinoma (HNSCC), combined programmed death receptor-1 inhibitor and chemotherapy improved response rate to neoadjuvant therapy. However, treatment response varies among patients. There is no tool to predict pathologic complete response (pCR) with high accuracy for now. To develop a tool based on radiomics features of MRI to predict pCR to neoadjuvant chemoimmunotherapy (NACI) may provide valuable assistance in treatment regimen determination for HNSCC.
Methods: From January 2021 to April 2024, a total of 172 patients with HNSCC from three medical center, who received NACI followed by surgery, were included and allocated into a training set (n=84), an internal validation set (n=37) and an external validation set (n=51). Radiomics features were extracted from intratumoral and different peritumoral areas, and radiomics signature (Rad-score) for each area was constructed. A radiomics-clinical nomogram was developed based on Rad-scores and clinicopathological characteristics, tested in the validation sets, and compared with clinical nomogram and combined positive score (CPS) in predicting pCR.
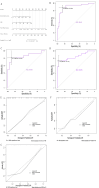
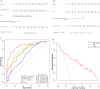
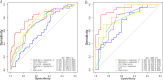
Results: The radiomics-clinical nomogram, incorporating peritumoral Rad-score, intratumoral Rad-score and CPS, achieved the highest accuracy with areas under the receiver operating characteristic curve of 0.904 (95% CI, 0.835 to 0.972) in the training cohort, 0.860 (95% CI, 0.722 to 0.998) in the internal validation cohort, and 0.849 (95% CI, 0.739 to 0.959) in the external validation cohort, respectively, which outperformed the clinical nomogram and CPS in predict pCR to NACI for HNSCC.
Conclusion: A nomogram developed based on intratumoral and peritumoral MRI radiomics features outperformed CPS, a widely employed biomarker, in predict pCR to NACI for HNSCC, which would provide incremental value in treatment regimen determination.
Keywords: Head and Neck Cancer; Immunotherapy; Neoadjuvant; Pathologic Complete Response - pCR.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures

References
-
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28. doi: 10.1016/S0140-6736(19)32591-7. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
