Input/Output Relationships for the Primary Hippocampal Circuit
- PMID: 39500578
- PMCID: PMC11713854
- DOI: 10.1523/JNEUROSCI.0130-24.2024
Input/Output Relationships for the Primary Hippocampal Circuit
Abstract
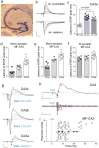
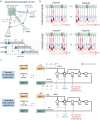
The hippocampus is the most studied brain region, but little is known about signal throughput-the simplest, yet most essential of circuit operations-across its multiple stages from perforant path input to CA1 output. Using hippocampal slices derived from male mice, we have found that single-pulse lateral perforant path (LPP) stimulation produces a two-part CA1 response generated by LPP projections to CA3 ("direct path") and the dentate gyrus ("indirect path"). The latter, indirect path was far more potent in driving CA1 but did so only after a lengthy delay. Rather than operating as expected from the much-discussed trisynaptic circuit argument, the indirect path used the massive CA3 recurrent collateral system to trigger a high-frequency sequence of fEPSPs and spikes. The latter events promoted reliable signal transfer to CA1, but the mobilization time for the stereotyped, CA3 response resulted in surprisingly slow throughput. The circuit transmitted theta (5 Hz) but not gamma (50 Hz) frequency input, thus acting as a low-pass filter. It reliably transmitted short bursts of gamma input separated by the period of a theta wave-CA1 spiking output under these conditions closely resembled the input signal. In all, the primary hippocampal circuit does not behave as a linear, three-part system but instead uses novel filtering and amplification steps to shape throughput and restrict effective input to select patterns. We suggest that the operations described here constitute a default mode for processing cortical inputs with other types of functions being enabled by projections from outside the extended hippocampus.
Keywords: amplifier; circuit analysis; hippocampus; lateral perforant path; low-pass filter; signal transformation.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Abeles M (1982) Role of the cortical neuron: integrator or coincidence detector? Isr J Med Sci 18:83–92. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
