Identifying WHO global priority endemic pathogens for vaccine research and development (R&D) using multi-criteria decision analysis (MCDA): an objective of the Immunization Agenda 2030
- PMID: 39500705
- PMCID: PMC11663754
- DOI: 10.1016/j.ebiom.2024.105424
Identifying WHO global priority endemic pathogens for vaccine research and development (R&D) using multi-criteria decision analysis (MCDA): an objective of the Immunization Agenda 2030
Abstract
Background: To date, global priorities for new vaccine R&D have not been systematically identified for endemic pathogens. As part of Immunisation Agenda 2030 (IA2030), we have systematically identified priority endemic pathogens for new vaccine R&D based on country and regional stakeholder values to address this need.
Methods: MCDA surveys targeting policy makers and immunisation stakeholders in each World Health Organization (WHO) region were used to weight eight criteria for prioritisation. Applying those weights to regional pathogen data yielded regional top ten pathogen lists, which are intended to inform regional deliberations on R&D priorities. The regional top ten lists were combined into an IA2030 global priority list. To inform R&D, use cases for new vaccines and monoclonal antibodies were identified, then categorized in terms of the activities needed to accelerate progress.
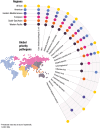
Findings: In five out of six WHO regions, Annual deaths in children under five and Contribution to antimicrobial resistance were the most heavily weighted criteria. How participants weighted the criteria was not associated with their region, biographical characteristics, or areas of expertise. Five pathogens were common priorities across all regions: M tuberculosis, HIV-1, K pneumoniae, S aureus, and Extra-intestinal pathogenic E coli. Six pathogens were priorities in single regions. Combining regional top ten lists provided a global list of 17 priority pathogens for new vaccine R&D. Thirty-four distinct use cases were identified for new products targeting these pathogens. While most are in the "Advance product development" category, ten are in the "Research" category and seven are in the "Prepare to implement" category.
Interpretation: These priorities for new vaccine R&D will help stakeholders better respond to regional and country needs. The use cases will inform R&D and enable monitoring of R&D under IA2030.
Funding: The work was funded by a Bill and Melinda Gates Foundation grant to WHO (INV-005318).
Keywords: Development; IA2030; Priorities; Research; Vaccines.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The paper declares no conflict of interest, but Angela Hwang, MS, and Kwaku Poku Asante, MD declare on their ICMJE forms consulting fees and support for attending meetings related to this research.
Figures

References
-
- O’Brien K.L., Binka F., Marsh K., Abramson J.S. Mind the gap: jumping from vaccine licensure to routine use. Lancet. 2016;387(10031):1887–1889. - PubMed
-
- World Health Organization . 2022. WHO R&D Blueprint for epidemics.www.who.int/teams/blueprint/who-r-and-d-blueprint-for-epidemics [cited 2024 Mar 26]. Available from:
-
- Tacconelli E., Carrara E., Savoldi A., et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. - PubMed
-
- World Health Organization . 2013. Global vaccine action plan 2011-2020 annex 1: recommended indicators.https://www.who.int/publications/i/item/global-vaccine-action-plan-2011-... [cited 2024 June 14]. Available from:
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

