Histone deacetylase 9 promotes osteogenic trans-differentiation of vascular smooth muscle cells via ferroptosis in chronic kidney disease vascular calcification
- PMID: 39500708
- PMCID: PMC11539403
- DOI: 10.1080/0886022X.2024.2422435
Histone deacetylase 9 promotes osteogenic trans-differentiation of vascular smooth muscle cells via ferroptosis in chronic kidney disease vascular calcification
Abstract
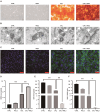
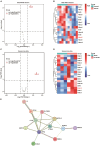
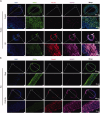
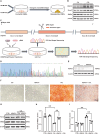
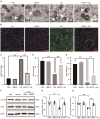
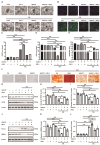
Vascular calcification, a common complication of chronic kidney disease (CKD), remains an unmet therapeutic challenge. The trans-differentiation of vascular smooth muscle cells (VSMCs) into osteoblast-like cells is crucial in the pathogenesis of vascular calcification in CKD. Despite ferroptosis promotes vascular calcification in CKD, the upstream or downstream regulatory mechanisms involved remains unclear. In this study, we aimed to investigate the regulatory mechanism involved in ferroptosis in CKD vascular calcification. Transcriptome sequencing revealed a potential relationship between HDAC9 and ferroptosis in CKD vascular calcification. Subsequently, we observed increased expression of HDAC9 in calcified arteries of patients undergoing hemodialysis and in a rat model of CKD. We further demonstrated that knockout of HDAC9 attenuates osteogenic trans-differentiation and ferroptosis in VSMCs stimulated by high calcium and phosphorus. In addition, RSL3 exacerbated ferroptosis and osteogenic trans-differentiation of VSMCs exposed to high levels of calcium and phosphorus, and these effects were suppressed to some extent by HDAC9 knockout. In summary, our findings suggest that HDAC9 promotes VSMCs osteogenic trans-differentiation involving ferroptosis, providing new insights for the therapy of CKD vascular calcification.
Keywords: HDAC9; ferroptosis; osteogenic trans-differentiation; vascular calcification; vascular smooth muscle cell.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Downregulation of HDAC9 by the ketone metabolite β-hydroxybutyrate suppresses vascular calcification.J Pathol. 2022 Nov;258(3):213-226. doi: 10.1002/path.5992. Epub 2022 Aug 31. J Pathol. 2022. PMID: 35894849
-
SREBP1 deficiency aggravates vascular calcification via iASPP-triggered ferroptosis of vascular smooth muscle cells.Cardiovasc Diabetol. 2025 Jun 3;24(1):236. doi: 10.1186/s12933-025-02797-3. Cardiovasc Diabetol. 2025. PMID: 40462076 Free PMC article.
-
p53 negatively regulates the osteogenic differentiation of vascular smooth muscle cells in mice with chronic kidney disease.Cardiovasc J Afr. 2012 Jul 12;23(6):e1-9. doi: 10.5830/CVJA-2011-069. Cardiovasc J Afr. 2012. PMID: 22143460 Free PMC article.
-
Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia.Cell Mol Life Sci. 2019 Jun;76(11):2077-2091. doi: 10.1007/s00018-019-03054-z. Epub 2019 Mar 18. Cell Mol Life Sci. 2019. PMID: 30887097 Free PMC article. Review.
-
Key regulators of vascular calcification in chronic kidney disease: Hyperphosphatemia, BMP2, and RUNX2.PeerJ. 2024 Sep 19;12:e18063. doi: 10.7717/peerj.18063. eCollection 2024. PeerJ. 2024. PMID: 39308809 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
