Efficacy and safety of JMT103 in patients with unresectable or surgically-challenging giant cell tumor of bone: a multicenter, phase Ib/II study
- PMID: 39500883
- PMCID: PMC11538294
- DOI: 10.1038/s41467-024-53686-4
Efficacy and safety of JMT103 in patients with unresectable or surgically-challenging giant cell tumor of bone: a multicenter, phase Ib/II study
Abstract
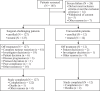
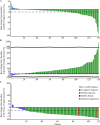
This was a multicenter, single-arm, open-label, phase Ib/II study (NCT04255576), aimed to evaluate the efficacy and safety of JMT103 in patients with unresectable or surgically-challenging giant cell tumor of bone (GCTB). JMT103 (2 mg/kg) was administered subcutaneously every four weeks, with loading doses on days 8 and 15. The primary endpoint was the objective tumor response rate (OTR) based on best response, defined as the proportion of patients who achieved elimination of at least 90% of the giant cells or radiologic complete or partial response per the modified Inverse Choi density/size (mICDS) or modified European Organization for Research and Treatment of Cancer (mEORTC) within 12 weeks. Secondary endpoints included objective response rate (ORR) per mICDS and mEORTC, and safety. A total of 139 patients were enrolled, and 135 were analyzed for efficacy. OTR, determined by the independent review committee (IRC) was 93.3% (95% CI 87.7-96.9). Treatment-related adverse events occurred in 90 (64.7%) patients, with hypophosphatemia and hypocalcemia being the most common. No serious treatment-related adverse events were observed. Thus, JMT103 demonstrates potential as a therapeutic option for GCTB.
© 2024. The Author(s).
Conflict of interest statement
J.Y. and H.L. are employees of the CSPC Pharmaceutical Group Limited. All other authors declare no competing interests.
Figures
References
-
- Mendenhall, W. M., Zlotecki, R. A., Scarborough, M. T., Gibbs, C. P. & Mendenhall, N. P. Giant cell tumor of bone. Am. J. Clin. Oncol.29, 96–99 (2006). - PubMed
-
- Montgomery, C., Couch, C., Emory, C. L. & Nicholas, R. Giant cell tumor of bone: review of current literature, evaluation, and treatment options. J. Knee Surg.32, 331–336 (2019). - PubMed
-
- The WHO Classification of Tumors Editorial Board. WHO Classification of Tumors Soft Tissue and Bone Tumors, 5th Edition edn.(IARC Press, 2020).
-
- Gamberi, G. et al. Identification of markers of possible prognostic value in 57 giant cell tumors of bone. Oncol. Rep.10, 351–356 (2003). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

