USP3 promotes DNA damage response and chemotherapy resistance through stabilizing and deubiquitinating SMARCA5 in prostate cancer
- PMID: 39500888
- PMCID: PMC11538284
- DOI: 10.1038/s41419-024-07117-3
USP3 promotes DNA damage response and chemotherapy resistance through stabilizing and deubiquitinating SMARCA5 in prostate cancer
Abstract
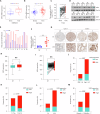
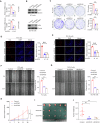
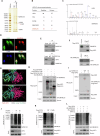
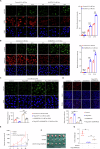
The chromatin-remodeling enzyme SMARCA5 plays a key role in DNA-templated events including transcription, DNA replication, and DNA repair. Loss of function of the SMARCA5 can cause neurodevelopmental disorder and Williams syndrome. However, the molecular mechanism underlying the regulation of SMARCA5 in prostate cancer remains largely elusive. Here, we report that the deubiquitinating enzyme USP3 directly interacts with SMARCA5 and removes K63-linked polyubiquitination of SMARCA5 to maintain its stability, which promotes DNA damage repair and chemotherapy resistance. Depletion of USP3 or SMARCA5 promoted PCa cells sensitive to docetaxel and overexpression of USP3 restored the cells resistance to docetaxel treatment in SMARCA5 silenced cells in vitro and vivo. Clinically, USP3 was significantly up-regulated in prostate cancer tissues and positively associated with SMARCA5 expression. Collectively, our findings uncover a novel molecular mechanism for the USP3-SMARCA5 axis in regulating DSB repair with an important role in chemotherapy response in human prostate cancers, highlighting that targeting USP3-SMARCA5 axis could be a valuable strategy to treat USP3/SMARCA5-overexpressing chemotherapy-resistant patients and improve drug treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Jiang X, Guo S, Wang S, Zhang Y, Chen H, Wang Y, et al. EIF4A3-Induced circARHGAP29 Promotes Aerobic Glycolysis in Docetaxel-Resistant Prostate Cancer through IGF2BP2/c-Myc/LDHA Signaling. Cancer Res. 2022;82:831–45. - PubMed
MeSH terms
Substances
Grants and funding
- Grant No. 82172921/National Natural Science Foundation of China (National Science Foundation of China)
- Grant No. 82260500/National Natural Science Foundation of China (National Science Foundation of China)
- grant no. 20151BAB205047/Natural Science Foundation of Jiangxi Province (Jiangxi Province Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

