Genomic variations associated with risk and protection against vincristine-induced peripheral neuropathy in pediatric cancer patients
- PMID: 39500896
- PMCID: PMC11538333
- DOI: 10.1038/s41525-024-00443-7
Genomic variations associated with risk and protection against vincristine-induced peripheral neuropathy in pediatric cancer patients
Abstract
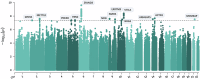
Vincristine-induced peripheral neuropathy is a common and highly debilitating toxicity from vincristine treatment that affects quality of life and often requires dose reduction, potentially affecting survival. Although previous studies demonstrated genetic factors are associated with vincristine neuropathy risk, the clinical relevance of most identified variants is limited by small sample sizes and unclear clinical phenotypes. A genome-wide association study was conducted in 1100 cases and controls matched by vincristine dose and genetic ancestry, uncovering a statistically significant (p < 5.0 × 10-8) variant in MCM3AP gene that substantially increases the risk of neuropathy and 12 variants protective against neuropathy within/near SPDYA, METTL8, PDE4D, FBN2, ZFAND3, NFIB, PAPPA, LRRTM3, NRG3, VTI1A, ARHGAP5, and ACTN1. A follow-up pathway analysis reveals the involvement of four key pathways, including nerve structure and development, myelination, neuronal transmission, and cytoskeleton/microfibril function pathways. These findings present potential actionable genomic markers of vincristine neuropathy and offer opportunities for tailored interventions to improve vincristine safety in children with cancer. This study is registered with ClinicalTrials.gov under the title National Active Surveillance Network and Pharmacogenomics of Adverse Drug Reactions in Children (ID NCT00414115, registered on December 21, 2006).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Pui, C.-H. & Evans, W. E. Treatment of acute lymphoblastic leukemia. N. Engl. J. Med.354, 166–178 (2006). - PubMed
-
- Said, R. & Tsimberidou, A. M. Pharmacokinetic evaluation of vincristine for the treatment of lymphoid malignancies. Expert Opin. Drug Metab. Toxicol.10, 483–494 (2014). - PubMed
-
- Pizzo, P.A. & Poplack, D.G. Principles and Practice of Pediatric Oncology. (Lippincott Williams & Wilkins, 2015).
-
- Gidding, C., Kellie, S., Kamps, W. & De Graaf, S. Vincristine revised. (1999). - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

