A bispecific CD40 agonistic antibody allowing for antibody-peptide conjugate formation to enable cancer-specific peptide delivery, resulting in improved T proliferation and anti-tumor immunity in mice
- PMID: 39500897
- PMCID: PMC11538452
- DOI: 10.1038/s41467-024-53839-5
A bispecific CD40 agonistic antibody allowing for antibody-peptide conjugate formation to enable cancer-specific peptide delivery, resulting in improved T proliferation and anti-tumor immunity in mice
Abstract
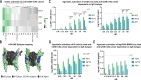
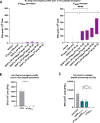
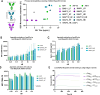
Current antibody-based immunotherapy depends on tumor antigen shedding for proper T cell priming. Here we select a novel human CD40 agonistic drug candidate and generate a bispecific antibody, herein named BiA9*2_HF, that allows for rapid antibody-peptide conjugate formation. The format is designed to facilitate peptide antigen delivery to CD40 expressing cells combined with simultaneous CD40 agonistic activity. In vivo, the selected bispecific antibody BiA9*2_HF loaded with peptide cargos induces improved antigen-specific proliferation of CD8+ (10-15 fold) and CD4+ T cells (2-7 fold) over control in draining lymph nodes. In both virus-induced and neoantigen-based mouse tumor models, BiA9*2_HF demonstrates therapeutic efficacy and elevated safety profile, with complete tumor clearance, as well as measured abscopal impact on tumor growth. The BiA9*2_HF drug candidate can thus be utilized to tailor immunotherapeutics for cancer patients.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: The presented study has been partly funded by Strike Pharma AB, whose long-term value can be influenced by the publication of the paper. S.M., J.R., A.M., I.L., M.L., and P.D. hold private stakes in Strike Pharma AB, whose long-term value can be influenced by the publication of the paper. R.V. is a current employee, and G.G.A. was an employee at Strike Pharma AB when the presented work was carried out. The rest of the authors declare no competing interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

