The power of 810 nm near-infrared photobiomodulation therapy for human asthenozoospermia
- PMID: 39501019
- PMCID: PMC11538380
- DOI: 10.1038/s41598-024-77823-7
The power of 810 nm near-infrared photobiomodulation therapy for human asthenozoospermia
Abstract
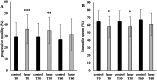
Sperm motility is a crucial factor in male fertility. Photobiomodulation (PBM) has been reported to increase sperm motility, but a consistent approach suitable for identifying standardizable protocols is lacking. We collected asthenozoospermic (n = 70) and normozoospermic (n = 20) semen. The asthenozoospermic samples were irradiated with an 810 nm diode laser, in continuous wave mode, at 0.25 W, 0.5 W, 1 W and 2 W for 60 s on a circular area of 1 cm2 through a novel handpiece with an innovative flat-top profile. Sperm motility was assessed immediately, after 30 and 60 min. A sample size calculator, unpaired t-test and one-way ANOVA with post-hoc Tukey HSD tests were used for statistics. One and 2 W were the most effective outputs in increasing progressive motility compared to control (p < 0.001). The maximum effect was immediately after 1 W-PBM (p < 0.001) and decreased after 60 min (p < 0.001). Time physiologically decreased vitality (p < 0.001), but less in the 1 W-PBM samples (p < 0.05). 1 W-PBM did not affect chromatin condensation. Asthenozoospermic samples displayed an impairment of 80% in oxygen consumption and ATP production and a slight inefficiency of oxidative phosphorylation compared to normozoospermic samples (p < 0.001). 1 W-PBM partially restored the functionality of aerobic metabolism (p < 0.001) by recovery of oxidative phosphorylation efficiency. PBM did not affect lactate dehydrogenase (glycolysis pathway). No irradiated samples increased accumulated malondialdehyde, a marker of lipidic peroxidation. In conclusion, PBM improves progressive motility in asthenozoospermia through increased mitochondrial energetic metabolism without harmful oxidative stress.
Keywords: Asthenozoospermia; DNA fragmentation; Low level laser therapy; Male fertility; Mitochondrial metabolism; Sperm motility; Sperm vitality.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Levine, H. et al. Temporal trends in sperm count: a systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum. Reprod. Update. 29, 157–176 (2023). - PubMed
-
- Amaral, A., Lourenço, B., Marques, M. & Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction. 146, R163–R174 (2013). - PubMed
-
- Boguenet, M., Bouet, P. E., Spiers, A. & Reynier, P. May-Panloup, P. Mitochondria: their role in spermatozoa and in male infertility. Hum. Reprod. Update. 27, 697–719 (2021). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

