Transuterine relocation of pregnant uterine horn segment in an exploratory rat model with implications for tubal ectopic pregnancy
- PMID: 39501022
- PMCID: PMC11538436
- DOI: 10.1038/s41598-024-76986-7
Transuterine relocation of pregnant uterine horn segment in an exploratory rat model with implications for tubal ectopic pregnancy
Abstract
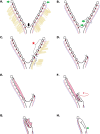
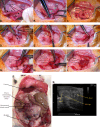
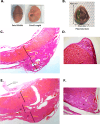
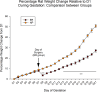
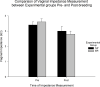
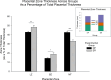
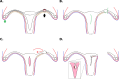
Ectopic pregnancy affects ~ 2% of pregnancies annually in the United States, with no current treatments allowing for the continuation of the pregnancy. Thus, this study sought to initiate an investigation into the potential design of a surgical technique, in an animal model, that could serve as a foundation for future research into the potential of relocating an ectopic embryo into the uterus at the human level. Female Long-Evans rats were randomly assigned to one of two groups: Embryo Relocation (ER; n = 12; underwent embryo relocation surgery) and Normal Pregnancy (NP; n = 12; carried a normal pregnancy; no surgery). Eight rats/group were allowed to carry their pregnancy to term and deliver, while four had their uteri collected at the end of gestation. Briefly, for the ER group, a uterine horn containing 1-2 embryos was translocated to the contralateral horn, which had been incised and cleared of its contents, prior to being wrapped around the relocated horn. Rat weight, food consumption and vaginal impedance of the mothers were measured throughout the experiment. Ultrasounds were performed and fetal heart rates measured on day 20-21 of gestation. Additionally, rat weight of all offspring was measured at adulthood. Our findings indicate that, in the ER group, 15/15 (100%) of the relocated embryos had detectable heart rates at the end of gestation (within the normal range), 14/15 (93%) were delivered vaginally, and 12/14 (86%) survived until adulthood. A significant decrease in rat weight and food consumption was observed only on the day following surgery. Fertility, as measured by vaginal impedance, was minimally impacted by surgery. Moreover, there was no significant difference between groups in average body weight of offspring at adulthood. Histological analysis indicated a thicker placenta in the ER group, attributable to the fetal part of the placenta, potentially indicating compensatory mechanisms. Our findings reflect a successful transuterine embryo relocation followed by vaginal birth and survival of offspring to adulthood, in a rat model. Such findings lay the foundation for future preclinical research in higher animals, with potential implications on a procedure relevant to human ectopic embryo relocation.
Keywords: Animal model; Ectopic pregnancy; Embryo relocation; Transplant; Tubal.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no financial and non-financial competing interests, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Figures
Similar articles
-
Surgical management of an ectopic pregnancy in the setting of an unexpected Müllerian anomaly: intraoperative and postoperative implications.Fertil Steril. 2024 Nov;122(5):951-953. doi: 10.1016/j.fertnstert.2024.07.036. Epub 2024 Jul 30. Fertil Steril. 2024. PMID: 39084353
-
Pregnancy-induced long-term uterine vascular remodeling in the rat.Reprod Biol. 2021 Mar;21(1):100466. doi: 10.1016/j.repbio.2020.100466. Epub 2020 Dec 3. Reprod Biol. 2021. PMID: 33279772
-
Ectopic pregnancy.Clin Obstet Gynecol. 1985 Jun;28(2):365-74. doi: 10.1097/00003081-198528020-00014. Clin Obstet Gynecol. 1985. PMID: 2410172
-
Interventions for non-tubal ectopic pregnancy.Cochrane Database Syst Rev. 2020 Jul 1;7(7):CD011174. doi: 10.1002/14651858.CD011174.pub2. Cochrane Database Syst Rev. 2020. PMID: 32609376 Free PMC article.
-
Conservative versus radical surgery for tubal pregnancy. A review.Acta Obstet Gynecol Scand. 1996 Jan;75(1):8-12. doi: 10.3109/00016349609033276. Acta Obstet Gynecol Scand. 1996. PMID: 8561006 Review.
References
-
- Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin 191: Tubal ectopic pregnancy. Obstet. Gynecol.131, e65–e77. 10.1097/AOG.0000000000002464 (2018). - PubMed
-
- Elson, J. et al. Expectant management of tubal ectopic pregnancy: prediction of successful outcome using decision tree analysis. Ultrasound Obstet. Gynecol.23, 552–556. 10.1002/uog.1061 (2004). - PubMed
-
- Helmy, S. et al. Fertility outcomes following expectant management of tubal ectopic pregnancy. Ultrasound Obstet. Gynecol.30, 988–993. 10.1002/uog.5186 (2007). - PubMed
-
- Bonin, L. et al. Predictive factors for the methotrexate treatment outcome in ectopic pregnancy: a comparative study of 400 cases. Eur. J. Obstet. Gynecol. Reprod. Biol.208, 23–30. 10.1016/j.ejogrb.2016.11.016 (2017). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

