A novel immunomodulating peptide with potential to complement oligodeoxynucleotide-mediated adjuvanticity in vaccination strategies
- PMID: 39501043
- PMCID: PMC11538426
- DOI: 10.1038/s41598-024-78150-7
A novel immunomodulating peptide with potential to complement oligodeoxynucleotide-mediated adjuvanticity in vaccination strategies
Abstract
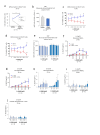
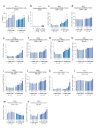
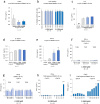
The identification of adjuvants to improve vaccination efficacy is a major unmet need. One approach is to augment the functionality of dendritic cells (DCs) by using Toll-like receptor-9 (TLR9) agonists such as cytosine-phosphate-guanine oligodeoxynucleotides (CpG ODNs) as adjuvants. Another approach is adjuvant selection based on production of bioactive interleukin-12 (IL-12). We report a D-peptide isomer, designated D-15800, that induces monocyte differentiation to the DC phenotype in vitro and more effectively stimulates IL-12p70 production upon T cell receptor (TCR) activation than the L-isomer. In the absence of TCR activation and either IL-12p70 or interleukin-2 production, only D-15800 activates CD4+ T and natural killer cells. In the presence of CpG ODN, D-15800 synergistically enhances production of interferon-alpha (IFN-α). Taken together with its biostability in human serum and depot retention upon injection, co-delivery of D-15800 with TLR9 agonists could serve to improve vaccine efficacy.
© 2024. The Author(s).
Conflict of interest statement
The authors M.A. and S.P. declare competing financial interest as Executive Directors of InterK Peptide Therapeutics Limited. The remaining authors do not have any competing interests.
Figures


References
-
- Fogel, M., Long, J. A., Thompson, P. J. & Upham, J. W. Dendritic cell maturation and IL-12 synthesis induced by the synthetic immune-response modifier S-28463. J. Leukoc. Biol.72, 932–938 (2002). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

