Structural variation reshapes population gene expression and trait variation in 2,105 Brassica napus accessions
- PMID: 39501128
- PMCID: PMC11549052
- DOI: 10.1038/s41588-024-01957-7
Structural variation reshapes population gene expression and trait variation in 2,105 Brassica napus accessions
Abstract
Although individual genomic structural variants (SVs) are known to influence gene expression and trait variation, the extent and scale of SV impact across a species remain unknown. In the present study, we constructed a reference library of 334,461 SVs from genome assemblies of 16 representative morphotypes of neopolyploid Brassica napus accessions and detected 258,865 SVs in 2,105 resequenced genomes. Coupling with 5 tissue population transcriptomes, we uncovered 285,976 SV-expression quantitative trait loci (eQTLs) that associate with altered expression of 73,580 genes. We developed a pipeline for the high-throughput joint analyses of SV-genome-wide association studies (SV-GWASs) and transcriptome-wide association studies of phenomic data, eQTLs and eQTL-GWAS colocalization, and identified 726 SV-gene expression-trait variation associations, some of which were verified by transgenics. The pervasive SV impact on how SV reshapes trait variation was demonstrated with the glucosinolate biosynthesis and transport pathway. The study highlighting the impact of genome-wide and species-scale SVs provides a powerful methodological strategy and valuable resources for studying evolution, gene discovery and breeding.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
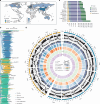






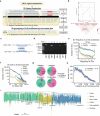






References
-
- Liu, Y. et al. Pan-genome of wild and cultivated soybeans. Cell182, 162–176 (2020). - PubMed
MeSH terms
Grants and funding
- U20A2034/National Natural Science Foundation of China (National Science Foundation of China)
- 32370681/National Natural Science Foundation of China (National Science Foundation of China)
- 32301868/National Natural Science Foundation of China (National Science Foundation of China)
- 32322061/National Natural Science Foundation of China (National Science Foundation of China)
- 32070559/National Natural Science Foundation of China (National Science Foundation of China)

