Comparison of three methods for generating the coccoid form of Helicobacter pylori and proteomic analysis
- PMID: 39501162
- PMCID: PMC11536543
- DOI: 10.1186/s12866-024-03599-5
Comparison of three methods for generating the coccoid form of Helicobacter pylori and proteomic analysis
Abstract
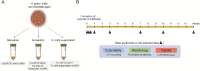
Background: Helicobacter pylori changes from spiral to coccoid depending on the host state, environmental factors, and surrounding microbial communities. The coccoid form of H. pylori still maintains its complete cellular structure, retains virulence genes, and thus plays a role in pathogenicity. To understand the coccoid form, it is crucial to establish the in vitro generation of the coccoid H. pylori. Although some conditions have been studied for the generation of the coccoid form, few studies have compared these conditions for coccoid generation. Here, we generated coccoid forms via three methods and compared the differences in morphology, viability, culturability, and protein expression.
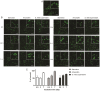
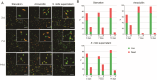
Results: The coccoid H. pylori was generated in vitro via three methods: a starvation method, a method using amoxicillin, and a method using the culture supernatant of Streptococcus mitis. The morphology and viability of the cells were examined by fluorescence microscopy after staining with SYTO9 and propidium iodide. The culturability of H. pylori was examined by counting colony-forming units on chocolate agar plates. In the starvation group, no colonies formed after 7 days, but viable coccoids were continuously observed. In the amoxicillin-treated group, the culturability decreased rapidly after 12 h, and showed a viable but non culturable (VBNC) state after the third day. Most cells treated with S. mitis supernatant changed to coccoid forms after 7 days, but colonies were continuously formed, probably due to living spiral forms. We performed proteomics to analyse the differences in protein profiles between the spiral and coccoid forms and protein profiles among the coccoid forms generated by the three methods.
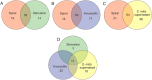
Conclusion: Amoxicillin treatment changed H. pylori to VBNC cells faster than starvation. Treatment with the S. mitis supernatant prolonged the culturability of H. pylori, suggesting that the S. mitis supernatant may contain substances that support spiral form maintenance. Proteomic analysis revealed that the expression of proteins differed between the spiral form and coccoid form of H. pylori, and this variation was observed among the coccoid forms produced via three different methods. The proteins in the coccoid forms produced by the three methods differed from each other, but common proteins were also observed among them.
Keywords: Helicobacter pylori; Streptococcus mitis; Amoxicillin; Coccoid form; Proteomics; Starvation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Assessment of cagE and babA mRNA expression during morphological conversion of Helicobacter pylori from spiral to coccoid.Curr Microbiol. 2013 Apr;66(4):406-13. doi: 10.1007/s00284-012-0280-7. Epub 2012 Dec 24. Curr Microbiol. 2013. PMID: 23263256
-
Understanding the dimorphic lifestyles of human gastric pathogen Helicobacter pylori using the SWATH-based proteomics approach.Sci Rep. 2016 May 25;6:26784. doi: 10.1038/srep26784. Sci Rep. 2016. PMID: 27222005 Free PMC article.
-
Overexpression of spoT gene in coccoid forms of clinical Helicobacter pylori isolates.Folia Microbiol (Praha). 2018 Jul;63(4):459-465. doi: 10.1007/s12223-017-0557-0. Epub 2018 Jan 11. Folia Microbiol (Praha). 2018. PMID: 29327293
-
[Helicobacter pylori morphological forms and their potential role in the transmission of infection].Postepy Hig Med Dosw (Online). 2014 Mar 4;68:219-29. doi: 10.5604/17322693.1092705. Postepy Hig Med Dosw (Online). 2014. PMID: 24662790 Review. Polish.
-
[Role of Helicobacter pylori coccoid forms in infection and recrudescence].Gastroenterol Hepatol. 2016 Jan;39(1):28-35. doi: 10.1016/j.gastrohep.2015.04.009. Epub 2015 Jun 15. Gastroenterol Hepatol. 2016. PMID: 26089229 Review. Spanish.
References
-
- Marshall B, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. lancet. 1984;323(8390):1311–5. - PubMed
-
- Blaser MJ. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102(2):720–7. 10.1016/0016-5085(92)90126-j. - PubMed
-
- Tominaga K, Hamasaki N, Watanabe T, Uchida T, Fujiwara Y, Takaishi O, et al. Effect of culture conditions on morphological changes of Helicobacter pylori. J Gastroenterol. 1999;34(Suppl 11):28–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

