Giardia antagonizes beneficial functions of indigenous and therapeutic intestinal bacteria during protein deficiency
- PMID: 39501168
- PMCID: PMC11542603
- DOI: 10.1080/19490976.2024.2421623
Giardia antagonizes beneficial functions of indigenous and therapeutic intestinal bacteria during protein deficiency
Abstract
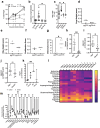
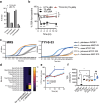
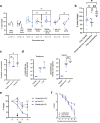
Undernutrition in children commonly disrupts the structure and function of the small intestinal microbial community, leading to enteropathies, compromised metabolic health, and impaired growth and development. The mechanisms by which diet and microbes mediate the balance between commensal and pathogenic intestinal flora remain elusive. In a murine model of undernutrition, we investigated the direct interactions Giardia lamblia, a prevalent small intestinal pathogen, on indigenous microbiota and specifically on Lactobacillus strains known for their mucosal and growth homeostatic properties. Our research reveals that Giardia colonization shifts the balance of lactic acid bacteria, causing a relative decrease in Lactobacillus spp. and an increase in Bifidobacterium spp. This alteration corresponds with a decrease in multiple indicators of mucosal and nutritional homeostasis. Additionally, protein-deficient conditions coupled with Giardia infection exacerbate the rise of primary bile acids and susceptibility to bile acid-induced intestinal barrier damage. In epithelial cell monolayers, Lactobacillus spp. mitigated bile acid-induced permeability, showing strain-dependent protective effects. In vivo, L. plantarum, either alone or within a Lactobacillus spp consortium, facilitated growth in protein-deficient mice, an effect attenuated by Giardia, despite not inhibiting Lactobacillus colonization. These results highlight Giardia's potential role as a disruptor of probiotic functional activity, underscoring the imperative for further research into the complex interactions between parasites and bacteria under conditions of nutritional deficiency.
Keywords: Giardia; bile acids; gnotobiotic models; malnourishment; probiotics.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Update of
-
Giardia Antagonizes Beneficial Functions of Indigenous and Therapeutic Intestinal Bacteria during Malnutrition.bioRxiv [Preprint]. 2024 Jan 23:2024.01.22.575921. doi: 10.1101/2024.01.22.575921. bioRxiv. 2024. Update in: Gut Microbes. 2024 Jan-Dec;16(1):2421623. doi: 10.1080/19490976.2024.2421623. PMID: 38328247 Free PMC article. Updated. Preprint.
References
-
- Roth DE, Krishna A, Leung M, Shi J, Bassani DG, Barros AJD.. Early childhood linear growth faltering in low-income and middle-income countries as a whole-population condition: analysis of 179 demographic and health surveys from 64 countries (1993–2015). Lancet Glob Health. 2017;5(12):e1249–24. doi:10.1016/S2214-109X(17)30418-7. - DOI - PMC - PubMed
-
- Vonaesch P, Araujo JR, Gody JC, Mbecko JR, Sanke H, Andrianonimiadana L, Naharimanananirina T, Ningatoloum SN, Vondo SS, Gondje PB, et al. Stunted children display ectopic small intestinal colonization by oral bacteria, which cause lipid malabsorption in experimental models. Proc Natl Acad Sci U S A. 2022;119(41):e2209589119. doi:10.1073/pnas.2209589119. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
