Association between toxin-antitoxin system mutations and global transmission of MDR-TB
- PMID: 39501228
- PMCID: PMC11539496
- DOI: 10.1186/s12879-024-10142-4
Association between toxin-antitoxin system mutations and global transmission of MDR-TB
Abstract
Background: The emergence of Multidrug-Resistant Tuberculosis (MDR-TB) poses a significant threat to global tuberculosis control efforts. This study aimed to examine the influence of mutations in Toxin-Antitoxin system genes on the global transmission of MDR-TB caused by Mycobacterium tuberculosis (M. tuberculosis).
Methods: Whole-genome sequencing was conducted on 13,518 M. tuberculosis isolates. Genes of the Toxin-Antitoxin system were obtained from the National Center for Biotechnology Information (NCBI) Gene database. Techniques such as Random Forest, Gradient Boosting Decision Tree, and Generalized Linear Mixed Models were employed to identify mutation sites in Toxin-Antitoxin system-related genes that facilitated the transmission of MDR-TB.
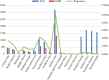
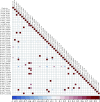
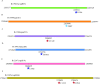
Results: 4,066 (30.08%) were identified as MDR-TB strains of all analyzed isolates. We found significant associations between specific gene mutations and MDR-TB transmission clusters including mutations in Rv0298 (G213A), Rv1959c (parE1, C88T), Rv1960c (parD1, C134T), Rv1991A (maze, G156A), Rv2547 (vapB, C54G), Rv2862A (vapB23, T2C), and Rv3385c (vapB46, G70A). Additionally, several gene mutations associated with MDR-TB transmission clades such as Rv1956 (higA, G445T), Rv1960c (parD1, C134T), and Rv1962A (vapB35, G99A) were noted. Certain gene mutations including vapB35 (G99A), higA (G445T), and parD1 (C134T) correlated with cross-regional transmission clades.
Conclusion: This study highlights the significant association between specific gene mutations in the Toxin-Antitoxin system and the global transmission of MDR-TB, providing valuable insights for developing targeted interventions to control MDR-TB.
Keywords: Gene mutations; Multidrug-resistant tuberculosis; Toxin-antitoxin system; Transmission.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Toxin-antitoxin system gene mutations driving Mycobacterium tuberculosis transmission revealed by whole genome sequencing.Front Microbiol. 2024 Jul 31;15:1398886. doi: 10.3389/fmicb.2024.1398886. eCollection 2024. Front Microbiol. 2024. PMID: 39144214 Free PMC article.
-
Molecular characterization of multidrug-resistant Mycobacterium tuberculosis (MDR-TB) isolates identifies local transmission of infection in Kuwait, a country with a low incidence of TB and MDR-TB.Eur J Med Res. 2019 Dec 5;24(1):38. doi: 10.1186/s40001-019-0397-2. Eur J Med Res. 2019. PMID: 31806020 Free PMC article.
-
Extensive global movement of multidrug-resistantM. tuberculosis strains revealed by whole-genome analysis.Thorax. 2019 Sep;74(9):882-889. doi: 10.1136/thoraxjnl-2018-211616. Epub 2019 May 2. Thorax. 2019. PMID: 31048508 Free PMC article.
-
Toxin-antitoxin Genes Expression in Multidrug-resistant Mycobacterium tuberculosis Isolates under Drug Exposure.Infect Disord Drug Targets. 2023;23(7):66-72. doi: 10.2174/1871526523666230524144448. Infect Disord Drug Targets. 2023. PMID: 37226796
-
Whole-genome sequencing of clinical isolates from tuberculosis patients in India: real-world data indicates a high proportion of pre-XDR cases.Microbiol Spectr. 2024 May 2;12(5):e0277023. doi: 10.1128/spectrum.02770-23. Epub 2024 Apr 10. Microbiol Spectr. 2024. PMID: 38597637 Free PMC article.
Cited by
-
Genomic characterization of multidrug-resistant tuberculosis in Shanghai, China: antibiotic resistance, virulence and transmission.JAC Antimicrob Resist. 2025 May 8;7(3):dlaf064. doi: 10.1093/jacamr/dlaf064. eCollection 2025 Jun. JAC Antimicrob Resist. 2025. PMID: 40342723 Free PMC article.
References
-
- World Health Organization. Global tuberculosis report 2023. Geneva: World Health Organization; 2023.
-
- T T, K G. Mechanism of post-segregational killing by the hok/sok system of plasmid R1. Sok antisense RNA regulates hok gene expression indirectly through the overlapping mok gene. Journal of molecular biology [Internet]. 1992 [cited 2024 Mar 25];223. https://pubmed.ncbi.nlm.nih.gov/1370544/ - PubMed
-
- Germain E, Castro-Roa D, Zenkin N, Gerdes K. Molecular mechanism of bacterial persistence by HipA. Mol Cell. 2013;52:248–54. - PubMed
-
- Harms A, Maisonneuve E, Gerdes K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science. 2016;354:aaf4268. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

