Western diet promotes endometriotic lesion growth in mice and induces depletion of Akkermansia muciniphila in intestinal microbiota
- PMID: 39501247
- PMCID: PMC11539706
- DOI: 10.1186/s12916-024-03738-9
Western diet promotes endometriotic lesion growth in mice and induces depletion of Akkermansia muciniphila in intestinal microbiota
Abstract
Background: Endometriosis, affecting 10% of women in their reproductive years, remains poorly understood. Both individual and environmental unexplained factors are implicated in this heterogenous condition. This study aims to examine the influence of a Western diet on endometriosis lesion development in mice and to uncover the mechanisms involved.
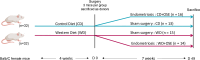
Methods: Mice were fed either a control diet or a Western diet (high in fatty acids and low in fiber) for 4 weeks. Endometriosis was then surgically induced, and lesion development was monitored by ultrasound. After 7 weeks, the mice were sacrificed for analysis of lesion characteristics through RT-qPCR, immunohistochemistry, and flow cytometry. Additionally, the intestinal microbiota was assessed using 16S rRNA gene sequencing.
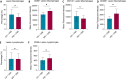
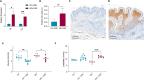
Results: Mice on the Western diet developed lesions that were significantly twice as large compared to those on the control diet. These lesions exhibited greater fibrosis and proliferation, alongside enhanced macrophage activity and leptin pathway expression. Changes in the intestinal microbiota were significantly noted after endometriosis induction, regardless of diet. Notably, mice on the Western diet with the most substantial lesions showed a loss of Akkermansia Muciniphila in their intestinal microbiota.
Conclusions: A Western diet significantly exacerbates lesion size in a mouse model of endometriosis, accompanied by metabolic and immune alterations. The onset of endometriosis also leads to substantial shifts in intestinal microbiota, suggesting a potential link between diet, intestinal health, and endometriosis development.
Keywords: Akkermansia muciniphila; Diet; Endometriosis; Intestinal Microbiota.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15:666–82. - PubMed
-
- Chapron C, Chopin N, Borghese B, Foulot H, Dousset B, Vacher-Lavenu MC, et al. Deeply infiltrating endometriosis: pathogenetic implications of the anatomical distribution. Hum Reprod Oxf Engl. 2006;21:1839–45. - PubMed
-
- Sampson JA. The development of the implantation theory for the origin of peritoneal endometriosis. Am J Obstet Gynecol. 1940;40:549–57.
-
- Christ JP, Yu O, Schulze-Rath R, Grafton J, Hansen K, Reed SD. Incidence, prevalence, and trends in endometriosis diagnosis: a United States population-based study from 2006 to 2015. Am J Obstet Gynecol. 2021;225:500.e1-500.e9. - PubMed
-
- Vallée A, Ceccaldi PF, Carbonnel M, Feki A, Ayoubi JM. Pollution and endometriosis: a deep dive into the environmental impacts on women’s health. BJOG Int J Obstet Gynaecol. 2024;131:401–14. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

