CD38 as theranostic target in oncology
- PMID: 39501292
- PMCID: PMC11539646
- DOI: 10.1186/s12967-024-05768-6
CD38 as theranostic target in oncology
Erratum in
-
Correction to: CD38 as theranostic target in oncology.J Transl Med. 2024 Dec 18;22(1):1113. doi: 10.1186/s12967-024-05959-1. J Transl Med. 2024. PMID: 39695745 Free PMC article. No abstract available.
Abstract
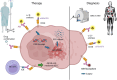
CD38 is a multifunctional transmembrane glycoprotein found in multiple tissues and overexpressed in many cancer cells, notably in hematological malignancies such as leukemia and multiple myeloma (MM). Therefore, targeting CD38 remains an attractive strategy for cancer treatment in hematological malignancies as well as in solid tumors. It plays a critical role in the progression of these diseases through its ADP-ribosyl cyclase and cADPR-hydrolase activities. Its importance has led to the development of various anti-CD38 monoclonal antibodies (mAbs), including daratumumab and isatuximab, approved for MM treatment. These mAbs exert their anti-tumor effects through Fc-dependent immune mechanisms and immunomodulation, enhancing T-cell and NK-cell-mediated responses. However, resistance mechanisms arise during the treatment with daratumumab, creating the necessity for new therapies. This review explains current knowledge about the role of CD38 as a target in oncology and aims to delineate the use of single domain antibodies (sdAbs) as innovative theranostic tools in nuclear medicine. For diagnostic purposes, PET radionuclides like 68 Ga, 64Cu, and SPECT radionuclides like 99mTc and 111In, are commonly used. Significant progress has been made in anti-CD38 radioligand therapy (RLT), with anti-CD38 antibodies providing insights into tumor biology and treatment efficacy. In terms of therapy, RLT is a promising approach that offers precise targeting of malignant cells while minimizing exposure to healthy tissue. This involves the use of radionuclides emitting α particles, like 225Ac, 212Pb or 211At, and β--particles like 90Y, 131I, or 177Lu, to exert cytotoxic effects. Derived from Camelidae heavy chain antibodies, sdAbs offer advantages over conventional mAbs such as small size, high stability, specificity, and ability to recognize hidden epitopes. CD38-specific sdAbs, such as sdAb 2F8, characterized by our laboratory, showing excellent tumor targeting and their engineered constructs, such as biparatopic antibodies and chimeric antibodies, represent a new generation of theranostic agents for diagnosis and treatment CD38-expressing malignancies.
© 2024. The Author(s).
Figures




References
-
- Graeff R, Liu Q, Kriksunov IA, Hao Q, Hon CL. Acidic residues at the active sites of CD38 and ADP-ribosylt cyclase determine nicotinic acid adenine dinucleotide phosphate (NAADP) synthesis and hydrolysis activities. J Biol Chem. 2006. 10.1074/jbc.M604370200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

