Comparative study of neutralizing antibodies titers in response to different types of COVID-19 vaccines among a group of egyptian healthcare workers
- PMID: 39501293
- PMCID: PMC11539826
- DOI: 10.1186/s12985-024-02546-0
Comparative study of neutralizing antibodies titers in response to different types of COVID-19 vaccines among a group of egyptian healthcare workers
Abstract
Background: Defining the protective thresholds against the severe-acute-respiratory-syndrome-related corona virus-2 pandemic is a crucial challenge. To reduce the risks of severe disease, hospitalization, and death, various COVID-19 vaccines have been rapidly developed.
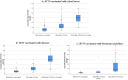
Aim of the work: This study aimed to assess the impact of three common COVID-19 vaccine types; two mRNA COVID-19 vaccines: (Pfizer/BioNTech's BNT162b2 and Moderna's mRNA-1273), one adenoviral vector vaccine: Oxford/AstraZeneca's ChAdOx1, and one inactivated vaccine (Sinovac Biotech/China's Sinovac) on the level of neutralizing antibodies, considering factors such as vaccine type, demographic characteristics, and hybrid immunity. We conducted a direct comparative analysis involving 300 healthcare workers, both with and without prior SARS-CoV-2 infection (B.1, C.36.3, and AY.32 (Delta) variants). Neutralizing antibodies levels were measured at baseline (before vaccination), before the second dose, and six months after the second dose.
Results: The results showed a significant increase in neutralizing antibodies levels after complete vaccination with all vaccine types. Among healthcare workers, those vaccinated with mRNA vaccines (Moderna or Pfizer) exhibited the highest neutralizing antibodies titers, followed by AstraZeneca, and finally Sinovac with the lowest titer. On studying the effect of previous COVID-19 infection after vaccination, no significant difference in neutralizing antibodies levels was observed between healthcare workers vaccinated with mRNA or AstraZeneca vaccines, both with prior COVID-19 infection, following the first and six months after the second dose.
Conclusion: These findings suggest that individuals with prior COVID-19 may only require a single dose of mRNA or AstraZeneca vaccines to achieve a similar level of immunization as those without prior COVID-19 who completed the vaccination program.
Highlights: There is a significant increase in neutralizing antibodies levels after complete vaccination against COVID-19 Vaccination with mRNA vaccines exhibits the highest neutralizing antibodies titers. Vaccination with Sinovac exhibits the lowest neutralizing antibodies titers.
Keywords: BNT162b2; COVID-19; ChAdOx1; Neutralizing antibodies; Sinovac.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard 2023. Cited 2023. Available from: https://covid19.who.int/. Accessed Sept 2023./vaccines?m49=818&n=c. Accessed March 18, 2024
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

