Optimizing CAR-T cell therapy for solid tumors: current challenges and potential strategies
- PMID: 39501358
- PMCID: PMC11539560
- DOI: 10.1186/s13045-024-01625-7
Optimizing CAR-T cell therapy for solid tumors: current challenges and potential strategies
Abstract
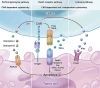
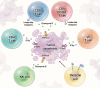
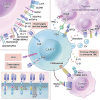
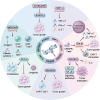
Chimeric antigen receptor (CAR)-T cell therapy demonstrates substantial efficacy in various hematological malignancies. However, its application in solid tumors is still limited. Clinical studies report suboptimal outcomes such as reduced cytotoxicity of CAR-T cells and tumor evasion, underscoring the need to address the challenges of sliding cytotoxicity in CAR-T cells. Despite improvements from fourth and next-generation CAR-T cells, new challenges include systemic toxicity from continuously secreted proteins, low productivity, and elevated costs. Recent research targets genetic modifications to boost killing potential, metabolic interventions to hinder tumor progression, and diverse combination strategies to enhance CAR-T cell therapy. Efforts to reduce the duration and cost of CAR-T cell therapy include developing allogenic and in-vivo approaches, promising significant future advancements. Concurrently, innovative technologies and platforms enhance the potential of CAR-T cell therapy to overcome limitations in treating solid tumors. This review explores strategies to optimize CAR-T cell therapies for solid tumors, focusing on enhancing cytotoxicity and overcoming application restrictions. We summarize recent advances in T cell subset selection, CAR-T structural modifications, infiltration enhancement, genetic and metabolic interventions, production optimization, and the integration of novel technologies, presenting therapeutic approaches that could improve CAR-T cell therapy's efficacy and applicability in solid tumors.
Keywords: CAR-T cell therapy; Challenges of cytotoxicity; Novel technologies; Restriction in application; Solid tumors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- 82270161/National Natural Science Foundation of China
- 2024B1515020054/Basic and Applied Basic Research Foundation of Guangdong Province
- 2023B1111050004/Science and Technology Planning Project of Guangdong Province
- KY0120220024 and KY012021160/NSFC Incubation Project of Guangdong Provincial People's Hospital
- DFJHBF202107/High-level Hospital Construction Project of Guangdong Provincial People's Hospital
LinkOut - more resources
Full Text Sources
Medical

