Harnessing the regenerative effects of human amniotic stem cells (hAFSCs) on restoring erectile function in a bilateral cavernous nerve crush (BCNC) injury rat model
- PMID: 39501401
- PMCID: PMC11539709
- DOI: 10.1186/s13287-024-03972-1
Harnessing the regenerative effects of human amniotic stem cells (hAFSCs) on restoring erectile function in a bilateral cavernous nerve crush (BCNC) injury rat model
Abstract
Background: Intracavernous (IC) injections of stem cells has been shown to ameliorate cavernous nerve (CN)-induced erectile dysfunction (ED). However, the regenerative effects underlying the recovery of erectile function (EF) in human amniotic fluid-derived stem cells (hAFSCs) remain unclear. In the bilateral cavernous nerve crushing (BCNC) injury rat paradigm, we sought to ascertain the effects of hAFSC treatment on EF recovery during the incipient phase.
Methods: Three groups of 45 male rats were used in this study: sham (Group 1), saline IC injection after BCNC (Group 2), and hAFSC intracavernous injection (ICI) after BCNC (Group 3). hAFSCs from the fourth passage showed potential to differentiate into trilineage cells. All animals were subjected to EF analysis on the 28th day post-injection and tissues were retrieved for histopathological and immunohistochemical analyses.
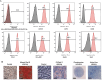
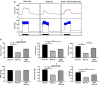
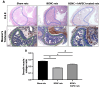
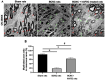
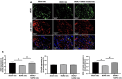
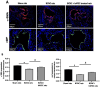
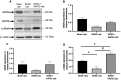
Results: IC injections of hAFSC significantly improved EF parameters in BCNC-ED rats at 28 days post-injury. AFSC treatment enhanced the smooth muscle condition and increased the smooth muscle/collagen ratio, as evidenced by histological analysis. Immunohistology revealed increased expression of 𝛼-SMA andvWf in the corpus cavernosum and enhanced expression of nNOS in the dorsal penile nerve in BCNC-ED rats (p < 0.05). Western blotting showed that hAFSC treatment significantly increased α-SMA expression in the hAFSC group compared with that in the BCNC group. Electron microscopy revealed significantly elevated myelination in the CN (p < 0.05), maintenance of smooth muscle structures, and restoration of EF in BCNC-ED rats treated with hAFSC.
Discussion and conclusions: hAFSC treatment increased EF in BCNC-ED rats at a single dose. As BCNC-ED resembles ED caused by radical prostatectomy (RP), this therapy has high potential for ED patients after RP surgery.
Keywords: BCNC injury; Electron microscopy; Erectile dysfunction; Stem cells; hAFSC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Liao CH, Chang CJ, Chen KC, Rajneesh CP, Tseng XW, Cheng JH, Chiang HS, Wu YN. Platelet-rich plasma glue placement at the prostatectomy site: effects on erectile function restoration and preservation of cavernous nerves in a rat model of nerve-sparing prostatectomy. Biomed Pharmacother. 2023;172:114499. 10.1016/j.biopha.2022.114499. - PubMed
-
- Irdam GA, Rasyid N, Taher A. Intracavernosal injection of mesenchymal stem cells for diabetic erectile dysfunction: a systematic review. Med J Indonesia. 2023;32(1):96–105. 10.13181/mji.v32i1.2549.
-
- Fandel TM, Albersen M, Lin G, Qiu X, Ning H, Banie L, Lue TF, Lin CS. Intracavernous injection of adipose-derived stem cells enhances erectile function in a rat model of cavernous nerve injury by recruiting cells to the major pelvic ganglion. Eur Urol. 2023;74(3):456–65. 10.1016/j.eururo.2022.08.036. - PMC - PubMed
-
- Mulhall JP, Smith AB. Penile rehabilitation following radical prostatectomy: a comprehensive review. Curr Opin Urol. 2006;16(6):410–4. 10.1016/j.biopha.2005.11.007. - PubMed
-
- Hatzimouratidis K, Salonia A. Pharmacotherapy for erectile dysfunction: recent advances and future prospects. J Sex Med. 2007;4(2):406–17. 10.1016/j.biopha.2006.07.004. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

