Oligodendrocyte Slc48a1 (Hrg1) encodes a functional heme transporter required for myelin integrity
- PMID: 39501820
- PMCID: PMC11662986
- DOI: 10.1002/glia.24641
Oligodendrocyte Slc48a1 (Hrg1) encodes a functional heme transporter required for myelin integrity
Abstract
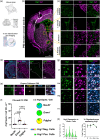
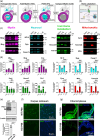
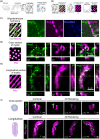
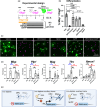
Oligodendrocytes (OLs) of the central nervous system require iron for proteolipid biosynthesis during the myelination process. Although most heme is found complexed to hemoglobin in red blood cells, surprisingly, we found that Slc48a1, encoding the heme transporter Hrg1, is expressed at higher levels in OLs than any other cell type in rodent and humans. We confirmed in situ that Hrg1 is expressed in OLs but not their precursors (OPCs) and found that Hrg1 proteins in CNS white matter co-localized within myelin sheaths. In older Hrg1 null mutant mice we observed reduced expression of myelin associated glycoprotein (Mag) and ultrastructural myelin defects reminiscent of Mag-null animals, suggesting myelin adhesion deficiency. Further, we confirmed reduced myelin iron levels in Hrg1 null animals in vivo, and show that OLs in vitro can directly import both the fluorescent heme analogue ZnMP and heme itself, which rescued iron deficiency induced inhibition of OL differentiation in a heme-oxidase-dependent manner. Together these findings indicate OL Hrg1 encodes a functional heme transporter required for myelin integrity.
Keywords: Hrg1; axon; heme; heme oxygenase; iron deficiency; myelin; myelin associated glycoprotein (Mag); myelin basic protein (Mbp); neurodegeneration; oligodendrocyte.
© 2024 The Author(s). GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
Iqbal Hamza is the President and Founder of Rakta Therapeutics Inc. (Maryland), a company involved in the development of heme transporter‐related diagnostics. He declares no other competing financial interests. The remaining authors declare no competing interests.
Figures



References
-
- Aboul‐Enein, F. , Rauschka, H. , Kornek, B. , Stadelmann, C. , Stefferl, A. , Brück, W. , Lucchinetti, C. , Schmidbauer, M. , Jellinger, K. , & Lassmann, H. (2003). Preferential loss of myelin‐associated glycoprotein reflects hypoxia‐like white matter damage in stroke and inflammatory brain diseases. Journal of Neuropathology and Experimental Neurology, 62, 25–33. - PubMed
-
- Alvarez, J. , Giuditta, A. , & Koenig, E. (2000). Protein synthesis in axons and terminals: Significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Progress in Neurobiology, 62, 1–62. - PubMed
-
- Andreini, C. , Putignano, V. , Rosato, A. , & Banci, L. (2018). The human iron‐proteome. Metallomics, 10, 1223–1231. - PubMed
-
- Asadollahi, E. , Trevisiol, A. , Saab, A. S. , Looser, Z. J. , Dibaj, P. , Ebrahimi, R. , Kusch, K. , Ruhwedel, T. , Möbius, W. , Jahn, O. , Lee, J. Y. , Don, A. S. , Khalil, M.‐A. , Hiller, K. , Baes, M. , Weber, B. , Abel, E. D. , Balabio, A. , Popko, B. , … Nave, K.‐A. (2024). Oligodendroglial fatty acid metabolism as a central nervous system energy reserve. Nature Neuroscience, 27, 1934–1944. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

