Robotic Assisted Ultrasound-Guided Endovascular Stent Implantation in a Vascular Model
- PMID: 39501897
- PMCID: PMC11670045
- DOI: 10.1002/rcs.70005
Robotic Assisted Ultrasound-Guided Endovascular Stent Implantation in a Vascular Model
Abstract
Background: Endovascular procedures are the preferred method for treating peripheral arterial disease. However, limited imaging options during these procedures, such as X-rays and contrast media, expose patients and healthcare professionals to potentially harmful radiation. This study introduces a robotic ultrasound system (RUSS) for navigating endovascular procedures in order to reduce radiation and provide additional information.
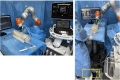
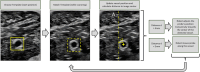
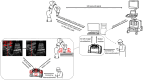
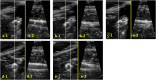
Methods: The RUSS comprises a seven-degree-of-freedom robotic arm that navigates an ultrasound transducer across a specific region of interest. The system is controlled by a self-programed software designed to navigate the robotic arm in a methodical and reproducible manner using a foot switch.
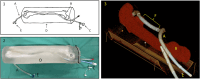
Results: An endovascular surgeon investigated the guidance and visibility of various guidewires and successfully implanted three stents in a vascular leg phantom using the RUSS without further radiation exposure.
Conclusions: The innovative set-up has several potential applications, including radiation-free endovascular procedures as well as health screening and diagnostic support in vascular medicine.
Keywords: 3D ultrasound; 3D vessel model; endovascular procedure without radiation; peripheral arterial disease (PAD); robotic ultrasound guidance; robotic ultrasound system (RUSS).
© 2024 The Author(s). The International Journal of Medical Robotics and Computer Assisted Surgery published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
The University of Luebeck holds a patent for the project under number DE 102020109593 B3, which is titled ‘Method for navigating an ultrasound augmented reality peripheral endovascular intervention and associated assembly for navigating an ultrasoundaugmented reality peripheral endovascular intervention’.
The University of Luebeck holds a patent for the project under number EP2737455 B1 in DE, GB and FR, filed in July 2011, under the title ‘Method for finding the position of a transducer’.
Figures

Similar articles
-
A Novel Endovascular Robotic System for Treatment of Lower Extremity Peripheral Arterial Disease: First-in-Human Experience.J Endovasc Ther. 2025 Feb;32(1):18-28. doi: 10.1177/15266028231182027. Epub 2023 Jul 7. J Endovasc Ther. 2025. PMID: 37415484
-
An augmented reality framework for optimization of computer assisted navigation in endovascular surgery.Annu Int Conf IEEE Eng Med Biol Soc. 2014;2014:5647-50. doi: 10.1109/EMBC.2014.6944908. Annu Int Conf IEEE Eng Med Biol Soc. 2014. PMID: 25571276
-
The role of robotic endovascular catheters in fenestrated stent grafting.J Vasc Surg. 2010 Apr;51(4):810-9; discussion 819-20. doi: 10.1016/j.jvs.2009.08.101. J Vasc Surg. 2010. PMID: 20347674
-
Current state in tracking and robotic navigation systems for application in endovascular aortic aneurysm repair.J Vasc Surg. 2015 Jan;61(1):256-64. doi: 10.1016/j.jvs.2014.08.069. Epub 2014 Oct 14. J Vasc Surg. 2015. PMID: 25441011 Review.
-
Current utilization and future directions of robotic-assisted endovascular surgery.Expert Rev Med Devices. 2020 Sep;17(9):919-927. doi: 10.1080/17434440.2020.1814742. Epub 2020 Aug 31. Expert Rev Med Devices. 2020. PMID: 32835546 Review.
References
-
- Bradbury A. W., Moakes C. A., Popplewell M., et al., “A Vein Bypass First Versus a Best Endovascular Treatment First Revascularisation Strategy for Patients With Chronic Limb Threatening Ischaemia Who Required an Infra‐popliteal, With or Without an Additional More Proximal Infra‐Inguinal Revascularisation Procedure to Restore Limb Perfusion (BASIL‐2): An Open‐Label, Randomised, Multicentre, Phase 3 Trial,” Lancet 401, no. 10390 (2023): 1798–1809, 10.1016/S0140-6736(23)00462-2. - DOI - PubMed
-
- Bolt L. J. J., Krasznai A. G., Sigterman T. A., Sikkink C. J. J. M., Schurink G. W. H., and Bouwman L. H., “Duplex‐Guided Versus Conventional Percutaneous Transluminal Angioplasty of Iliac TASC II A and B Lesion: A Randomized Controlled Trial,” Annals of Vascular Surgery 55 (2019): 138–147, 10.1016/J.AVSG.2018.07.047. - DOI - PubMed
-
- Ascher E., Marks N. A., Hingorani A. P., Schutzer R. W., and Mutyala M., “Duplex‐Guided Endovascular Treatment for Occlusive and Stenotic Lesions of the Femoral‐Popliteal Arterial Segment: A Comparative Study in the First 253 Cases,” Journal of Vascular Surgery 44, no. 6 (2006): 1230–1237, 10.1016/J.JVS.2006.08.025. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

