Myofibroblasts persist through immune privilege mechanisms to mediate oral submucous fibrosis: Uncovering the pathogenesis
- PMID: 39502133
- PMCID: PMC11535754
- DOI: 10.1016/j.jobcr.2024.10.008
Myofibroblasts persist through immune privilege mechanisms to mediate oral submucous fibrosis: Uncovering the pathogenesis
Abstract
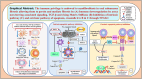
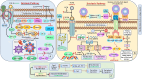
Immune privilege is the ability to tolerate foreign antigens without eliciting an inflammatory immune response. Several mechanisms explain a structure's immune privilege status, which is regulated by innate and adaptive immune responses. The role of myofibroblasts in perpetuating fibrosis by acquiring an immune privileged phenotype against the backdrop of oral submucous fibrosis (OSF) is evolving. Myofibroblasts persist through the Fas/FasL autocrine pathway and induce apoptosis in epithelial cells, explaining the juxtaposition of apoptotic cells in areas of fibrosis. However, increased matrix stiffness, in addition to activating TGF-β, reduces Fas surface expression in myofibroblasts, increasing their resistance to apoptosis. The reciprocal amplification loop between the immune checkpoint proteins programmed death-ligand 1 (PD-L1) and TGF-β involves the YAP-TAZ and SMAD2,3 pathways and dramatically enhances profibrotic signalling. Increased matrix stiffness also enhances cMYC expression, which subsequently amplifies PD-L1 levels on myofibroblasts. The increase in PD-L1 on the myofibroblast microengineers the phenotype of CD4+ T cells homing to fibrotic areas by acting on the programmed cell death protein 1 (PD-1) receptor on the T-cell surface, converting these cells from antifibrotic cells to profibrotic cells that produce IL-17A and TGF-β. This manuscript provides mechanistic insight into how myofibroblasts avoid apoptosis in OSFs by evading the immune system. Targeting an immune-privileged phenotype in myofibroblasts with FAS-FASL pathway-dependent characteristics is an ideal strategy for reversing OSF.
Keywords: Fas-FasL pathway; Immune checkpoint protein; Myofibroblast; Oral submucous fibrosis; PD1-PD-L1 pathway; Tregs.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Gayathri K., Malathi N., Gayathri V., Adtani P.N., Ranganathan K. Molecular pathways of oral submucous fibrosis and its progression to malignancy. Arch Oral Biol. 2023;148 - PubMed
-
- Sharma M., Shetty S.S., Radhakrishnan R. Oral submucous fibrosis as an overhealing wound: implications in malignant transformation. Recent Pat Anti-Cancer Drug Discov. 2018;13:272–291. - PubMed
-
- Dvorak H.F. Tumors: wounds that do not heal-A historical perspective with a focus on the fundamental roles of increased vascular permeability and clotting. Semin Thromb Hemost. 2019;45:576–592. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

