The implications of oncolytic viruses targeting fibroblasts in enhancing the antitumoural immune response
- PMID: 39502212
- PMCID: PMC11535324
- DOI: 10.1016/j.heliyon.2024.e39204
The implications of oncolytic viruses targeting fibroblasts in enhancing the antitumoural immune response
Abstract
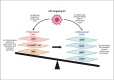
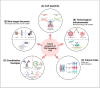
Oncolytic viruses (OVs) are an emerging immunotherapy platform that selectively target tumour cells, inducing immunogenic cell death. This reverses the 'immune-desert' phenotype of tumours, enhancing antitumour immunity. However, oncolytic virotherapy has shown limited efficacy in solid tumours due to the presence of protumoural, immunosuppressive cancer-associated fibroblasts (CAFs). Recent studies have explored OVs that specifically target CAFs to enhance antitumoural immune responses, with promising results. Nevertheless, detailed interrogation of the experimental design of these studies casts doubt on their potential for successful clinical translation. Most studies targeted CAFs non-specifically, failing to acknowledge CAF heterogeneity, with antitumoural CAFs also present. Thus, use of transcriptomics is advisable to provide more focused targeting, limiting potential off-target toxicity. Furthermore, experiments to date have largely been conducted in murine models that do not faithfully recapitulate tumour microenvironments, potentially biasing the efficacy observed. Future work should make use of humanised patient-derived xenograft murine models for animal studies, after which primary human tumour biopsies should be utilised to more closely represent the patient population for maximal translation relevance. Additionally, approaches to enhance the antitumoural immune responses of this therapy should be prioritised, with the ultimate aim of achieving complete remission, which has not yet been observed pre-clinically.
Keywords: Anti-tumoural immunity; Cancer-associated fibroblast; Immunotherapy; Oncolytic virus.
© 2024 The Author(s).
Conflict of interest statement
None.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

