Assessing the biobehavioral effects of ultramicronized-palmitoylethanolamide monotherapy in autistic adults with different severity levels: a report of two cases
- PMID: 39502301
- PMCID: PMC11536324
- DOI: 10.3389/fpsyt.2024.1463849
Assessing the biobehavioral effects of ultramicronized-palmitoylethanolamide monotherapy in autistic adults with different severity levels: a report of two cases
Abstract
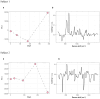
Despite promise of its supplementation as both monotherapy and add-on treatment in autism spectrum disorder (ASD), the biobehavioral effects of Palmitoylethanolamide (PEA) in autistic adults have never been explored so far. We discussed the cases of two autistic adults with different degrees of severity (level 1 and level 2) presenting with symptoms of psychic distress, who were treated with ultramicronized-PEA (um-PEA) 600 mg/day monotherapy for a sustained period of 4 months. The level 1 autistic patient showed improved depressive symptoms and social engagement at a 12-week follow-up, in parallel to a tendency toward reduced inflammatory response and enhanced endocannabinoid (eCB) signaling, partially relapsing after um-PEA discontinuation at four months. Opposedly, the level 2 autistic patient exhibited a generally stable psychosocial functioning for the initial 12 weeks, consistent with basically unchanged immune and eCBs levels, abruptly deteriorating and leading to antipsychotic initiation afterwards. No significant side effects were reported in both cases during the observation period. The two cases suggest that um-PEA could be an effective option for the treatment of psychic distress in level 1 autistic adults, warranting further investigation of its age- and level-specificity and of the biological underpinnings of its therapeutic effect in ASD.
Keywords: cannabinoids; entourage effect; glutamate signaling; neurodevelopmental disorders; nutraceutical; peroxisome proliferator activated receptor alpha; supplementary food.
Copyright © 2024 Bortoletto, Piscitelli, Basaldella, Scipioni, Comacchio, Fiorino, Fornasaro, Barbieri, Pagliaro, Sepulcri, Fabris, Curcio, Balestrieri and Colizzi.
Conflict of interest statement
MC has been a consultant and advisor to GW Pharma Limited, F. Hoffmann-La Roche Limited, and GW Pharma Italy SRL outside of this work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. FP and MC declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th. Washington, DC: American Psychiatric Association (APA) (2013) p. 50–9.
Publication types
LinkOut - more resources
Full Text Sources