Changes in 24-hour blood pressure profile after 12 weeks of dapagliflozin treatment in patients with diabetic kidney disease: an Italian multicenter prospective study
- PMID: 39502370
- PMCID: PMC11536757
- DOI: 10.1093/ckj/sfae316
Changes in 24-hour blood pressure profile after 12 weeks of dapagliflozin treatment in patients with diabetic kidney disease: an Italian multicenter prospective study
Abstract
Background: Sodium-glucose cotransporter 2 inhibitors (SGLT2i) lower ambulatory blood pressure (ABP) in patients with type 2 diabetes mellitus; whether the same holds true in diabetic kidney disease (DKD) is unknown. This information is critical to the knowledge of mechanisms of nephroprotection and safety of this therapy.
Methods: This multicenter prospective study evaluates the changes in ABP after 12 weeks of dapagliflozin 10 mg/day in a cohort of patients with type 2 DKD and glomerular filtration rate (GFR) >25 mL/min/1.73 m2. Primary endpoint was the change of nighttime systolic blood pressure (SBP). Changes of daytime SBP, prevalence of normal dipping (day/night SBP ratio <0.9) and changes in ABP patterns, that is, sustained uncontrolled hypertension (SUCH), white coat uncontrolled hypertension (WUCH), masked uncontrolled hypertension (MUCH) and controlled hypertension (CH) were secondary endpoints.
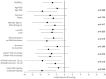
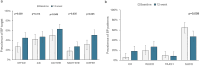
Results: Eighty-three of 96 patients completed the study [age 68.7 ± 8.9 years, 73.5% males, GFR 49 ± 17 mL/min/1.73 m2, median albuminuria: 0.18 (interquartile range 0.10-0.38) g/24 h]. After 12 weeks of dapagliflozin, nighttime SBP declined by -3.0 mmHg (95% confidence interval -5.2/-0.8 mmHg; P = .010) with an improvement of nighttime SBP goal (<110 mmHg) from 18.0% to 27.0% (P < .001). Similarly, the prevalence of normal dipping increased (from 31.3% to 50.6%, P = .005). A decrease in daytime (-2.4 mmHg; P = .046) and office (-7.9 mmHg; P = .009) SBP was also found. The decline of ambulatory and office SBP was associated with increased prevalence of CH (from 6.0% to 18.0%) and significant improvement of SUCH, WUCH and MUCH (P = .009). Albuminuria decreased (P < .001), whereas eGFR did not change (P = .297). Urinary tract infection (4.2%) and acute kidney injury (3.6%) were the main causes of drop-out. Only one patient showed a drop of nighttime SBP below 90 mmHg.
Conclusions: Dapagliflozin is associated with improvement in circadian blood pressure rhythm with no major safety signal related to excessive blood pressure decrease.
Keywords: SGLT2 inhibitors; ambulatory blood pressure; diabetes kidney disease; nocturnal hypertension.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
A.C., C.G. and D.I. have no conflict to disclose. S.B. has received fees for lectures by AstraZeneca, Baxter, Mayoli and Vifor Pharma. L.D.N. has received fees for scientific consultation and/or lectures by Astellas, AstraZeneca, Mundibiopharma and Vifor Pharma. R.M. has been member of Advisory Boards for Astellas, Bayer, GSK and Amgen, and has been an invited speaker at meetings supported by Amgen, Astellas and Vifor Pharma. M.C. is member of the CKJ Editorial Board and has been member of Advisory Boards for Astellas, Bayer, GSK, Vifor Pharma and Amgen, and has been an invited speaker at meetings supported by Amgen, Astellas and Vifor Pharma. Filippo Au.: fees for lectures and scientific consultations by CSL-Vifor, AstraZeneca, Bayer.
Figures

References
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2022;102:S1–127. - PubMed
-
- Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium . Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 2022;400:1788–801. 10.1016/S0140-6736(22)02074-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

