The role of baseline 18F-FDG PET/CT for survival prognosis in NSCLC patients undergoing immunotherapy: a systematic review and meta-analysis
- PMID: 39502406
- PMCID: PMC11536524
- DOI: 10.1177/17588359241293364
The role of baseline 18F-FDG PET/CT for survival prognosis in NSCLC patients undergoing immunotherapy: a systematic review and meta-analysis
Abstract
Background: The value of pretreatment baseline 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET)/computed tomography (CT) as a prognostic factor for survival of patients with non-small-cell lung cancer (NSCLC) receiving immunotherapy remained uncertain.
Objectives: To investigate the prognostic ability of baseline 18F-FDG PET/CT in patients with NSCLC receiving immunotherapy.
Design: A systematic review and meta-analysis.
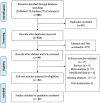
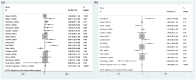
Data sources and methods: We searched the PubMed, EMBASE, and Cochrane Central Register of Controlled Trials databases until May 7, 2024, and extracted data related to patient characteristics, semiquantitative parameters of 18F-FDG PET/CT, and survival. We pooled hazard ratios (HRs) to evaluate the prognostic value of the maximum standardized uptake value (SUVmax), mean standardized uptake value (SUVmean), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) for overall survival (OS) and progression-free survival (PFS).
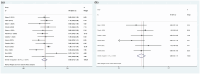
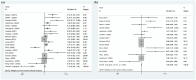
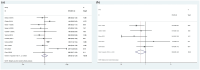
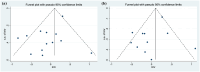
Results: A total of 22 studies (1363 patients, average age range 30-88 years) were included. Baseline 18F-FDG PET/CT-derived MTV was significantly associated with both OS (HR: 1.124, 95% confidence interval (CI) 1.058-1.195, I 2 = 81.70%) and PFS (HR: 1.069, 95% CI: 1.016-1.124, I 2 = 71.80%). Other baseline 18F-FDG PET/CT-derived parameters, including SUVmax (OS: HR: 0.930, 95% CI: 0.718-1.230; PFS: HR: 0.979, 95% CI: 0.759-1.262), SUVmean (OS: HR: 0.801, 95% CI: 0.549-1.170; PFS: HR: 0.688, 95% CI: 0.464-1.020), and TLG (OS: HR: 0.999, 95% CI: 0.980-1.018; PFS: HR: 0.995, 95% CI: 0.980-1.010), were not associated with survival. Sensitivity analyses by removing one study at a time did not significantly alter the association between MTV and PFS or between MTV and OS. There was no evidence of publication bias.
Conclusion: Pretreatment baseline 18F-FDG PET/CT-derived MTV might be a prognostic biomarker in NSCLC patients receiving immunotherapy. Further studies are needed to support routine use.
Keywords: 18F-FDG PET/CT imaging; PD-1/PD-L1; immune checkpoint inhibitor; immunotherapy; non-small-cell lung cancer; response assessment.
Plain language summary
Using PET/CT scans to predict survival in lung cancer patients receiving immunotherapy: a study review Aims and Purpose of the Research We wanted to know if a type of scan called 18F-FDG PET/CT can help predict how long people with a type of lung cancer (NSCLC) will live after treatment with immunotherapy. Background of the Research This research matters because NSCLC is a common and serious type of lung cancer. Knowing how long patients might live after treatment can help doctors plan better care. Many people are affected by this disease, so finding good ways to predict survival can help a lot of patients. Methods and Research Design They reviewed and analyzed data from 22 different studies involving 1363 patients, with ages ranging from 30 to 88 years.We focused on certain measurements from the scans, like SUVmax, SUVmean, MTV, and TLG. We checked if these measurements were linked to how long patients lived and how long they lived without their cancer getting worse. Results and Importance We found that one of these measurements, the Metabolic Tumor Volume (MTV), was linked to how long the patients lived and how long they stayed free of disease after treatment. Specifically, higher MTV was associated with poorer overall survival and progression-free survival. The other measurements (SUVmax, SUVmean, and TLG) did not show a significant connection to patient survival. In conclusion, the MTV from PET/CT scans might help doctors predict the outcomes for lung cancer patients undergoing immunotherapy. However, more studies are needed to confirm these findings and to consider using this measurement regularly in clinical practice.
© The Author(s), 2024.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
-
- The Lancet. Lung cancer: some progress, but still a lot more to do. Lancet (London, England) 2019; 394(10212): 1880. - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin 2021; 71(1): 7–33. - PubMed
-
- Travis WD, Brambilla E, Burke AP, et al. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol 2015; 10(9): 1240–1242. - PubMed
-
- Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA 2019; 322(8): 764–774. - PubMed
-
- Mazzone P, Mekhail T. Current and emerging medical treatments for non-small cell lung cancer: a primer for pulmonologists. Respir Med 2012; 106(4): 473–492. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

