Unveiling six novel bacterial strains for fipronil and thiobencarb biodegradation: efficacy, metabolic pathways, and bioaugmentation potential in paddy soil
- PMID: 39502414
- PMCID: PMC11536974
- DOI: 10.3389/fmicb.2024.1462912
Unveiling six novel bacterial strains for fipronil and thiobencarb biodegradation: efficacy, metabolic pathways, and bioaugmentation potential in paddy soil
Abstract
Introduction: Soil bacteria offer a promising approach to bioremediate pesticide contamination in agricultural ecosystems. This study investigated the potential of bacteria isolated from rice paddy soil for bioremediating fipronil and thiobencarb, common agricultural pesticides.
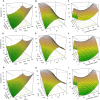
Methods: Bacterial isolates capable of degrading fipronil and thiobencarb were enriched in a mineral salt medium. A response surface methodology with a Box-Behnken design was utilized to optimize pesticide degradation with the isolated bacteria. Bioaugmentation tests were performed in paddy soils with varying conditions.
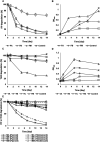
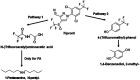
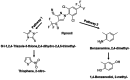
Results and discussion: Six strains, including single isolates and their mixture, efficiently degraded these pesticides at high concentrations (up to 800 µg/mL). Enterobacter sp., Brucella sp. (alone and combined), and a mixture of Stenotrophomonas sp., Bordetella sp., and Citrobacter sp. effectively degraded fipronil and thiobencarb, respectively. Notably, a single Pseudomonas sp. strain degraded a mixture of both pesticides. Optimal degradation conditions were identified as a slightly acidic pH (6-7), moderate pesticide concentrations (20-50 µg/mL), and a specific inoculum size. Bioaugmentation assays in real-world paddy soils (sterile/non-sterile, varying moisture) demonstrated that these bacteria significantly increased degradation rates (up to 14.15-fold for fipronil and 5.13-fold for thiobencarb). The study identifies these novel bacterial strains as promising tools for bioremediation and bioaugmentation strategies to tackle fipronil and thiobencarb contamination in paddy ecosystems.
Keywords: bacteria; bioremediation; degradation rate; pesticide; response surface methodology; transformation products.
Copyright © 2024 Faridy, Torabi, Pourbabaee, Osdaghi and Talebi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Abraham J., Silambarasan S., Logeswari P. (2014). Simultaneous degradation of organophosphorus and organochlorine pesticides by bacterial consortium. J. Taiwan Inst. Chem. Eng. 45, 2590–2596. doi: 10.1016/j.jtice.2014.06.014 - DOI
-
- Anastassiades M., Lehotay S. J., Štajnbaher D., Schenck F. J. (2003). Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 86, 412–431. doi: 10.1093/jaoac/86.2.412, PMID: - DOI - PubMed
-
- Anderson R. T., Vrionis H. A., Ortiz-Bernad I., Resch C. T., Long P. E., Dayvault R., et al. . (2003). Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69, 5884–5891. doi: 10.1128/AEM.69.10.5884-5891.2003, PMID: - DOI - PMC - PubMed
-
- Bhatt P., Rene E. R., Kumar A. J., Gangola S., Kumar G., Sharma A., et al. . (2021a). Fipronil degradation kinetics and resource recovery potential of Bacillus sp. strain FA4 isolated from a contaminated agricultural field in Uttarakhand, India. Chemosphere 276:130156. doi: 10.1016/j.chemosphere.2021.130156 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

