Distinction of pseudoprogression from true progression in glioblastomas using machine learning based on multiparametric magnetic resonance imaging and O6-methylguanine-methyltransferase promoter methylation status
- PMID: 39502470
- PMCID: PMC11535496
- DOI: 10.1093/noajnl/vdae159
Distinction of pseudoprogression from true progression in glioblastomas using machine learning based on multiparametric magnetic resonance imaging and O6-methylguanine-methyltransferase promoter methylation status
Abstract
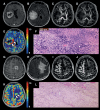
Background: It is imperative to differentiate true progression (TP) from pseudoprogression (PsP) in glioblastomas (GBMs). We sought to investigate the potential of physiologically sensitive quantitative parameters derived from diffusion and perfusion magnetic resonance imaging (MRI), and molecular signature combined with machine learning in distinguishing TP from PsP in GBMs in the present study.
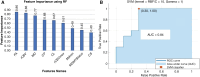
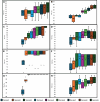
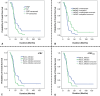
Methods: GBM patients (n = 93) exhibiting contrast-enhancing lesions within 6 months after completion of standard treatment underwent 3T MRI. Final data analyses were performed on 75 patients as O6-methylguanine-DNA-methyltransferase (MGMT) status was available only from these patients. Subsequently, patients were classified as TP (n = 55) or PsP (n = 20) based on histological features or mRANO criteria. Quantitative parameters were computed from contrast-enhancing regions of neoplasms. PsP datasets were artificially augmented to achieve balanced class distribution in 2 groups (TP and PsP). A random forest algorithm was applied to select the optimized features. The data were randomly split into training and testing subsets in an 8:2 ratio. To develop a robust prediction model in distinguishing TP from PsP, several machine-learning classifiers were employed. The cross-validation and receiver operating characteristic (ROC) curve analyses were performed to determine the diagnostic performance.
Results: The quadratic support vector machine was found to be the best classifier in distinguishing TP from PsP with a training accuracy of 91%, cross-validation accuracy of 86%, and testing accuracy of 85%. Additionally, ROC analysis revealed an accuracy of 85%, sensitivity of 70%, and specificity of 100%.
Conclusions: Machine learning using quantitative multiparametric MRI may be a promising approach to distinguishing TP from PsP in GBMs.
Keywords: diffusion and perfusion MR imaging; glioblastoma; machine-learning; pseudoprogression; true progression.
© The Author(s) 2024. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
The authors declare no conflict of interest. The authors have no relevant financial or nonfinancial interests to disclose.
Figures

References
-
- Gunjur A, Lau E, Taouk Y, Ryan G.. Early post-treatment pseudo-progression amongst glioblastoma multiforme patients treated with radiotherapy and temozolomide: A retrospective analysis. J Med Imaging Radiat Oncol. 2011;55(6):603–610. - PubMed
-
- Van Mieghem E, Wozniak A, Geussens Y, et al. Defining pseudoprogression in glioblastoma multiforme. Eur J Neurol. 2013;20(10):1335–1341. - PubMed
-
- Kong DS, Kim ST, Kim EH, et al. Diagnostic dilemma of pseudoprogression in the treatment of newly diagnosed glioblastomas: The role of assessing relative cerebral blood flow volume and oxygen-6-Methylguanine-DNA Methyltransferase Promoter Methylation Status. AJNR Am J Neuroradiol. 2011;32(2):382–387. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
