New insights into the roles of lactylation in cancer
- PMID: 39502530
- PMCID: PMC11534861
- DOI: 10.3389/fphar.2024.1412672
New insights into the roles of lactylation in cancer
Abstract
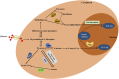
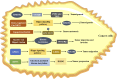
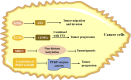
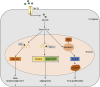
Lactylation, a novel discovered posttranslational modification, is a vital component of lactate function and is prevalent in a wide range of cells, interacting with both histone and non-histone proteins. Recent studies have confirmed that lactylation as a new contributor to epigenetic landscape is involved in multiple pathological processes. Accumulating evidence reveals that lactylation exists in different pathophysiological states and leads to inflammation and cancer; however, few mechanisms of lactylation have been elaborated. This review summarizes the biological processes and pathophysiological roles of lactylation in cancer, as well as discusses the relevant mechanisms and potential therapeutic targets, aiming to provide new insights for targeted cancer therapy.
Keywords: cancer; histone and non-histone proteins; lactate; lactylation; therapeutic targets.
Copyright © 2024 Zhu, Liu, Luo, Xiao and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bennis Y., Bodeau S., Batteux B., Gras-Champel V., Masmoudi K., Maizel J., et al. (2020). A study of associations between plasma metformin concentration, lactic acidosis, and mortality in an emergency hospitalization context. Crit. Care Med. 48, e1194–e1202. 10.1097/CCM.0000000000004589 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

