Risk Classification of Patients With Advanced Urothelial Carcinoma Treated With Enfortumab Vedotin
- PMID: 39502620
- PMCID: PMC11534046
- DOI: 10.21873/cdp.10396
Risk Classification of Patients With Advanced Urothelial Carcinoma Treated With Enfortumab Vedotin
Abstract
Background/aim: Enfortumab Vedotin (EV) is a widely used antibody-drug conjugate for patients with advanced urothelial carcinoma (UC) who have previously been treated with platinum-based chemotherapy and immune checkpoint inhibitors. However, limited information is currently available on prognostic factors and risk classification. Therefore, the present study attempted to identify clinical factors that predict outcomes in patients with advanced UC treated with EV and to develop a novel risk classification model.
Patients and methods: We conducted a multicenter retrospective study including patients with advanced UC treated with EV. Oncological outcomes were assessed using progression-free survival (PFS) and overall survival (OS), and prognostic factors for PFS and OS were investigated. We then examined the usefulness of risk classification based on the prognostic factors identified.
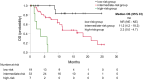
Results: Median PFS and OS were 7.1 and 16.3 months, respectively. High C-reactive protein levels (CRP level ≥0.5 mg/dl) and hypercalcemia (corrected calcium level >10.2 mg/dl) were identified as prognostic factors for PFS (p=0.012 and p=0.003, respectively) and OS (p=0.035 and p<0.001, respectively). We then divided patients into three risk groups: no prognostic factors group, one prognostic factor group, and two prognostic factors group. Significant differences were observed in PFS and OS among the three groups (p<0.001 and p<0.001, respectively) and c-indices were 0.766 for PFS and 0.800 for OS.
Conclusion: The risk classification using CRP and hypercalcemia is useful for predicting the outcomes of patients with advanced UC treated with EV.
Keywords: Enfortumab Vedotin; prognostic factor; risk classification.
©2024 The Author(s). Published by the International Institute of Anticancer Research.
Conflict of interest statement
The Authors declare no conflicts of interest in relation to this study.
Figures
References
-
- Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF, KEYNOTE-045 Investigators Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. - DOI - PMC - PubMed
-
- Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, Kalofonos H, Radulović S, Demey W, Ullén A, Loriot Y, Sridhar SS, Tsuchiya N, Kopyltsov E, Sternberg CN, Bellmunt J, Aragon-Ching JB, Petrylak DP, Laliberte R, Wang J, Huang B, Davis C, Fowst C, Costa N, Blake-Haskins JA, di Pietro A, Grivas P. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. - DOI - PubMed
-
- Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Durán I, Lee JL, Matsubara N, Vulsteke C, Castellano D, Wu C, Campbell M, Matsangou M, Petrylak DP. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384(12):1125–1135. doi: 10.1056/NEJMoa2035807. - DOI - PMC - PubMed
-
- Fukuokaya W, Koike Y, Yata Y, Komura K, Uchimoto T, Tsujino T, Saruta M, Takahara K, Fujita K, Minami T, Adachi T, Hirasawa Y, Hashimoto T, Ohno Y, Uemura H, Shiroki R, Azuma H, Kimura T. Real world evidence of enfortumab vedotin in patients with advanced urothelial cancer: A multicenter observational study. Int J Urol. 2024;31(4):342–347. doi: 10.1111/iju.15368. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous