This is a preprint.
The role of the microbiota in respiratory virus-bacterial pathobiont relationships in the upper respiratory tract
- PMID: 39502658
- PMCID: PMC11537323
- DOI: 10.1101/2024.10.22.24315478
The role of the microbiota in respiratory virus-bacterial pathobiont relationships in the upper respiratory tract
Update in
-
Role of the upper airway microbiota in respiratory virus and bacterial pathobiont dynamics in the first year of life.Nat Commun. 2025 Jun 4;16(1):5195. doi: 10.1038/s41467-025-60552-4. Nat Commun. 2025. PMID: 40467627 Free PMC article.
Abstract
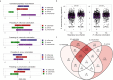
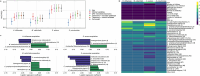
The mechanisms by which respiratory viruses predispose to secondary bacterial infections remain poorly characterized. Using 2,409 nasopharyngeal swabs from 300 infants in Botswana, we performed a detailed analysis of factors that influence the dynamics of bacterial pathobiont colonization during infancy. We quantify the extent to which viruses increase the acquisition of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae. We provide evidence of cooperative interactions between these pathobionts while identifying host characteristics and environmental exposures that influence the odds of pathobiont colonization during early life. Using 16S rRNA gene sequencing, we demonstrate that respiratory viruses result in losses of putatively beneficial Corynebacterium and Streptococcus species that are associated with a lower odds of pathobiont acquisition. These findings provide novel insights into viral-bacterial relationships in the URT of direct relevance to respiratory infections and suggest that the URT bacterial microbiota is a potentially modifiable mechanism by which viruses promote bacterial respiratory infections.
Keywords: Haemophilus influenzae; Moraxella catarrhalis; Staphylococcus aureus; Streptococcus pneumoniae carriage; childhood respiratory infections; children; nasopharyngeal microbiota; respiratory microbiome; sub-Saharan Africa.
Conflict of interest statement
Competing interests: MSK is a consultant for Merck & Co, Inc. and Invivyd. All other authors declare that they have no competing interests.
Figures


References
-
- Bender RG, Sirota SB, Swetschinski LR, Dominguez R-MV, Novotney A, Wool EE, et al. Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies, 1990–2021: a systematic analysis from the Global Burden of Disease Study 2021. The Lancet Infectious Diseases. 2024. - PMC - PubMed
-
- Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. The Lancet Global Health. 2018;6(7):e744–e57. - PMC - PubMed
-
- Chien Y-W, Klugman KP, Morens DM. Bacterial pathogens and death during the 1918 influenza pandemic. New England Journal of Medicine. 2009;361(26):2582–3. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
