Leishmania major surface components and DKK1 signalling via LRP6 promote migration and longevity of neutrophils in the infection site
- PMID: 39502693
- PMCID: PMC11534728
- DOI: 10.3389/fimmu.2024.1473133
Leishmania major surface components and DKK1 signalling via LRP6 promote migration and longevity of neutrophils in the infection site
Abstract
Background: Host-related factors highly regulate the increased circulation of neutrophils during Leishmania infection. Platelet-derived Dickkopf-1 (DKK1) is established as a high-affinity ligand to LRP6. Recently, we demonstrated that DKK1 upregulates leukocyte-platelet aggregation, infiltration of neutrophils to the draining lymph node and Th2 differentiation during Leishmania infection, suggesting the potential involvement of the DKK1-LRP6 signalling pathway in neutrophil migration in infectious diseases.
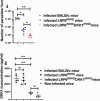
Results: In this study, we further explored the potential role of DKK1-LRP6 signalling in the migration and longevity of activated neutrophils in the infection site using BALB/c mice with PMNs deficient in LRP6 (LRP6NKO) or BALB/c mice deficient in both PMN LRP6 and platelet DKK1 (LRP6NKO DKK1PKO). Relative to the infected wild-type BALB/c mice, reduced neutrophil activation at the infection site of LRP6NKO or LRP6NKO DKK1PKO mice was noted. The neutrophils obtained from either infected LRP6NKO or LRP6NKO DKK1PKO mice additionally showed a high level of apoptosis. Notably, the level of LRP6 expressing neutrophils was elevated in infected BALB/c mice. Relative to infected BALB/c mice, a significant reduction in parasite load was observed in both LRP6NKO and LRP6NKO DKK1PKO infected mice. Notably, DKK1 levels were comparable in the LRP6NKO and BALB/c mice in response to infection, indicating that PMN activation is the major pathway for DKK1 in promoting parasitemia. Parasite-specific components also play a crucial role in modulating neutrophil circulation in Leishmania disease. Thus, we further determine the contribution of Leishmania membrane components in the migration of neutrophils to the infection site using null mutants deficient in LPG synthesis (Δlpg1- ) or lacking all ether phospholipids (plasmalogens, LPG, and GIPLs) synthesis (Δads1- ). Relative to the WT controls, Δads1- parasite-infected mice showed a sustained decrease in neutrophils and neutrophil-platelet aggregates (for at least 14 days PI), while neutrophils returned to normal in Δlpg1- parasite-infected mice after day 3 PI.
Conclusion: Our results suggest that DKK1 signalling and Leishmania pathogen-associated molecular patterns appear to regulate the migration and sustenance of viable activated neutrophils in the infection site resulting in chronic type 2 cell-mediated inflammation.
Keywords: apoptosis; innate response; leishmaniasis; neutrophils; platelet.
Copyright © 2024 Ihedioha, Marcarian, Sivakoses, Beverley, McMahon-Pratt and Bothwell.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




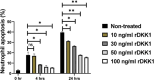
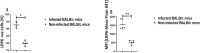
Similar articles
-
CCL7 Is a Negative Regulator of Cutaneous Inflammation Following Leishmania major Infection.Front Immunol. 2019 Jan 8;9:3063. doi: 10.3389/fimmu.2018.03063. eCollection 2018. Front Immunol. 2019. PMID: 30671055 Free PMC article.
-
Toll-like receptor 2 (TLR2) plays a role in controlling cutaneous leishmaniasis in vivo, but does not require activation by parasite lipophosphoglycan.Parasit Vectors. 2016 Oct 6;9(1):532. doi: 10.1186/s13071-016-1807-8. Parasit Vectors. 2016. PMID: 27716391 Free PMC article.
-
An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major.J Immunol. 2000 Sep 1;165(5):2628-36. doi: 10.4049/jimmunol.165.5.2628. J Immunol. 2000. PMID: 10946291
-
Distinct immunological states in murine cutaneous leishmaniasis by immunising with different amounts of antigen: the generation of beneficial, potentially harmful, harmful and potentially extremely harmful states.Behring Inst Mitt. 1997 Feb;(98):153-9. Behring Inst Mitt. 1997. PMID: 9382736 Review.
-
Anti-leishmania effector functions of CD4+ Th1 cells and early events instructing Th2 cell development and susceptibility to Leishmania major in BALB/c mice.Adv Exp Med Biol. 1998;452:53-60. doi: 10.1007/978-1-4615-5355-7_7. Adv Exp Med Biol. 1998. PMID: 9889959 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

