Acute-phase innate immune responses in SIVmac239-infected Mamu-B*08+ Indian rhesus macaques may contribute to the establishment of elite control
- PMID: 39502699
- PMCID: PMC11534762
- DOI: 10.3389/fimmu.2024.1478063
Acute-phase innate immune responses in SIVmac239-infected Mamu-B*08+ Indian rhesus macaques may contribute to the establishment of elite control
Abstract
Introduction: Spontaneous control of chronic-phase HIV/SIV viremia is often associated with the expression of specific MHC class I allotypes. HIV/SIV-specific CD8+ cytotoxic T lymphocytes (CTLs) restricted by these MHC class I allotypes appear to be critical for viremic control. Establishment of the elite controller (EC) phenotype is predictable in SIVmac239-infected Indian rhesus macaques (RMs), with approximately 50% of Mamu-B*08+ RMs and 20% of Mamu-B*17+ RMs becoming ECs. Despite extensive characterization of EC-associated CTLs in HIV/SIV-infected individuals, the precise mechanistic basis of elite control remains unknown. Because EC and non-EC viral load trajectories begin diverging by day 14 post-infection, we hypothesized that hyperacute innate immune responses may contribute to viremic control.
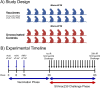
Methods: To gain insight into the immunological factors involved in the determination of EC status, we vaccinated 16 Mamu-B*08+ RMs with Vif and Nef to elicit EC-associated CTLs, then subjected these 16 vaccinees and an additional 16 unvaccinated Mamu-B*08+ controls to repeated intrarectal SIVmac239 challenges. We then performed whole-blood transcriptomic analysis of all 32 SIVmac239-infected Mamu-B*08+ RMs and eight SIVmac239-infected Mamu-B*08 - RMs during the first 14 days of infection.
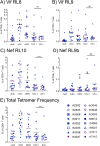
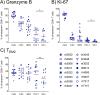
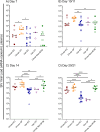
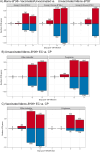
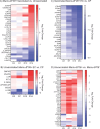
Results: Vaccination did not provide protection against acquisition, but peak and setpoint viremia were significantly lower in vaccinees relative to controls. We did not identify any meaningful correlations between vaccine-induced CTL parameters and SIVmac239 acquisition rate or chronic-phase viral loads. Ultimately, 13 of 16 vaccinees (81%) and 7 of 16 controls (44%) became ECs (viremia ≤ 10,000 vRNA copies/mL plasma for ≥ 4 weeks). We identified subsets of immunomodulatory genes differentially expressed (DE) between RM groupings based on vaccination status, EC status, and MHC class I genotype. These DE genes function in multiple innate immune processes, including the complement system, cytokine/chemokine signaling, pattern recognition receptors, and interferon-mediated responses.
Discussion: A striking difference in the kinetics of differential gene expression among our RM groups suggests that Mamu-B*08-associated elite control is characterized by a robust, rapid innate immune response that quickly resolves. These findings indicate that, despite the association between MHC class I genotype and elite control, innate immune factors in hyperacute SIV infection preceding CTL response development may facilitate the establishment of the EC phenotype.
Keywords: acquired immunodeficiency syndrome (AIDS); cytotoxic T lymphocytes (CTLs); human immunodeficiency virus (HIV); simian immunodeficiency virus (SIV); vaccines.
Copyright © 2024 Rosen, Sawatzki, Ricciardi, Smith, Golez, Mauter, Pedreño-López, Yrizarry-Medina, Weisgrau, Vosler, Voigt, Louw, Tisoncik-Go, Whitmore, Panayiotou, Ghosh, Furlott, Parks, Desrosiers, Lifson, Rakasz, Watkins and Gale.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

